- Omicron Variant, thymol, gingerol, salvinorina A, cynnamil, curcumin, pulegone, rosmarinic acid
Copyright (c) 2022 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
The goal of this paper was determining the physical and chemical properties of some medicinal plants which are used against the Omicron Variant (Covid-19 variant B.1.1.529) symptoms. In this work, seven medicinal species for the most frequently symptoms of Omicron disease such as fever, cough, sore throat, shortness of breath, anorexia, muscle-joint pain, headache and Nausea-vomiting based on the fidelity level index has been accomplished.
Positive stranded RNA viruses, coronaviruses (CoVs), can infect humans and multiple species of animals through enteric, respiratory, and central nervous system diseases with attractive targets for designing anti- Omicron conjunction. In this work, it has been investigated the compounds of thymol, gingerol, salvinorina A, cynnamil, curcumin, pulegone and rosmarinic acid as a probable anti pandemic Omicron receptor derived from medicinal plants and herbs of thyme, ginger , salvia divinorum, cinnamon leaves, curcuma longa (turmeric) , mentha pulegium (pennyroyal) and rosemary, respectively.
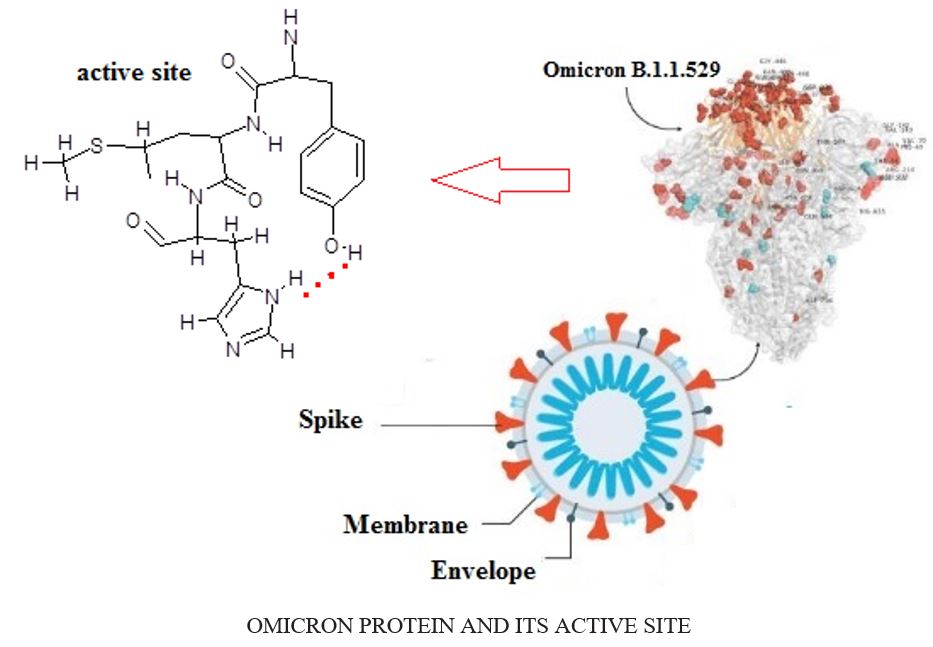
Anti-Omicron through the hydrogen bonding using the physicochemical properties including heat of formation, Gibbs free energy, electronic energy, charge distribution of active parts in the hydrogen bonding, NMR estimation of medicinal ingredients jointed to the database amino acids fragment of Tyr-Met-His as the selective zone of the Omicron, positive frequency and intensity of different normal modes of these structures have been evaluated. The theoretical calculations were done at various levels of theory to gain the more accurate equilibrium geometrical results, and IR spectral data for each of the complex proposed drugs of N-terminal or O-terminal auto-cleavage substrate were individually determined to elucidate the structural flexibility and substrate binding of seven medicinal plants jointed to active site of Omicron molecule. A comparison of these structures with two configurations provides new insights for the design of substrate-based anti-targeting Omicron. This indicates a feasible model for designing wide-spectrum of anti- Omicron drugs.
The structure-based optimization of these structures has yielded two more efficacious lead compounds, N and O atoms through forming the hydrogen bonding (H-bonding) with potent anti- Omicron Variant (Covid-19 variant B.1.1.529) .
References
- Zhang, J.; Zhou, L.; Yang, Y.; Peng, W.; Wang, W.; Chen, X. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet 2020, 395, e39. https://doi.org/10.1016/S0140-6736(20)30313-5.
- Pandey, U.; Greninger, A.L.; Levin, G.R. Pathogen or bystander: clinical significance of detecting human herpesvirus 6 in pediatric cerebrospinal fluid. J Clin Microbiol 2020, 58, e00313-e00320. https://doi.org/10.1128/JCM.00313-20.
- Mollaamin,F.; Esmkhani,R.; Monajjemi,M. Mutations in Novel COVID-19 Make it More Dangerous: Prevention Via Scientific Approaches. Biointerface Res. Appl. Chem2021, 11(3), 10546-10558. https://doi.org/10.33263/BRIAC113.1054610558.
- Yan, B.; Chu, H.; Yang, D.; Sze, K.-H.; Lai, P.-M.; Yuan, S.; Shuai, H.; Wang, Y.; Kao, R.Y.-T.; Chan, J.F.-W.; Yuen, K.-Y. Characterization of the Lipidomic Profile of Human Coronavirus-Infected Cells: Implications for Lipid Metabolism Remodeling upon Coronavirus Replication. Viruses 2019, 11,73. https://doi.org/10.3390/v11010073.
- Fouchier, R. A.M.; Kuiken, T.; Schutten,M.; van Amerongen,G.; van Doornum, G.J.J.; van den Hoogen, B.G.; Peiris,M.; Lim,W.; Stohr,K.; and Osterhaus, A.D.M.E. Aetiology: Koch’s postulates fulfilled for SARS virus. Nature 2003, 423,240. https://doi.org/10.1038/423240a.
- Ksiaze k, T. G.; Erdman,D.; Goldsmith, C. S.; Zaki, S. R.; Peret,T.; Emery,S.; Tong,S.; Urbani,C.; Comer,J.A.; Lim,W.; Rollin,P.E.; Dowell, S.F.; Ling,A.E.; Humphrey, C.D.; Shieh, W.J.; Guarner,J.; Paddock,C.D.; Rota,P.; Fields,B.; DeRisi,J.; Yang, J.Y.; Cox,N.; Hughes, J.M.; LeDuc, J.W.; Bellini, W.J.; and Anderson. L.J. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med 2003, 348, 1953-1966. https://doi.org/10.1056/NEJMoa030781.
- Shi, C.S.; Nabar, N.R.; Huang, N.N.; et al. SARS-Coronavirus Open reading frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019;5:101. https://doi.org/10.1038/s41420-019-0181-7.
- Mollaamin,F. Function of Anti-CoV Structure Using INH [1-6]- Tyr160-Met161-His162 Complex. Biointerface Res. Appl. Chem2021, 11(6), 14433 - 14450. https://doi.org/10.33263/BRIAC116.1443314450.
- Mitton, B.; Rule, R.; Said, M. Laboratory evaluation of the BioFire FilmArray Pneumonia plus panel compared to conventional methods for the identification of bacteria in lower respiratory tract specimens: a prospective cross-sectional study from South Africa. Diagn Microbiol Infect Dis 2021, 99, 115236. https://doi.org/10.1016/j.diagmicrobio.2020.115236.
- Kao,C.-C.; Chiang,H.-T.; Chen,C.-Y.; Hung,C.-T.; Chen,Y.-C.; Su,L.-H.; Shi,Z.-Y. et al. National bundle care program implementation to reduce ventilator-associated pneumonia in intensive care units in Taiwan. J Microbiol Immunol Infect 2019, 52, 592-597. https://doi.org/10.1016/j.jmii.2017.11.001.
- Yen, M.Y.; Schwartz, J.; Chen, S.Y.; King, C.C.; Yang, G.Y.; Hsueh, P.R. Interrupting COVID-19 transmission by implementing enhanced traffic control bundling: Implications for global prevention and control efforts. J Microbiol Immunol Infect 2020, 53,377-380. https://doi.org/10.1016/j.jmii.2020.03.011.
- Álvarez-Lerma, F.; Sánchez García, M.; The multimodal approach for ventilator-associated pneumonia prevention-requirements for nationwide implementation. Ann Transl Med 2018, 6, 420. https://doi.org/10.21037/atm.2018.08.40.
- Caméléna, F.; Moy, A.C.; Dudoignon, E.; Poncin, T.; Deniau, B.; Guillemet, L.; Le Goff, J. et al. Diagn Microbiol Infect Dis 2021, 99, 115183. https://doi.org/10.1016/j.diagmicrobio.2020.115183.
- Dien Bard, J.; McElvania, E. Panels and Syndromic Testing in Clinical Microbiology. Clin Lab Med 2020, 40, 393-420. https://doi.org/10.1016/j.cll.2020.08.001.
- Van, T.T.; Kim,T.H.; Butler-Wu, S.M. Evaluation of the Biofire FilmArray meningitis/encephalitis assay for the detection of Cryptococcus neoformans/gattii. Clin Microbiol Infect 2020, S1198-743X, 30031-30038. https://doi.org/10.1016/j.cmi.2020.01.007.
- Tansarli, G.S.; Chapin, K.C. Diagnostic test accuracy of the BioFire(R) FilmArray(R) meningitis/encephalitis panel: a systematic review and meta-analysis. Clin Microbiol Infect 2020, 26, 281-290. https://doi.org/10.1016/j.cmi.2019.11.016.
- Nabower, A.M.; Miller, S.; Biewen,B. Association of the FilmArray meningitis/encephalitis panel with clinical management. Hosp Pediatr. 2019,9,763-769. https://doi.org/10.1542/hpeds.2019-0064.
- Cailleaux, M.; Pilmis, B.; Mizrahi, A. Impact of a multiplex PCR assay (FilmArray(R)) on the management of patients with suspected central nervous system infections. Eur J Clin Microbiol Infect Dis 2020, 39, 293-297. https://doi.org/10.1007/s10096-019-03724-7.
- She, R.C.; Bender, J.M. Advances in rapid molecular blood culture diagnostics: healthcare impact, laboratory implications, and multiplex technologies. J Appl Lab Med 2019, 3, 617-630. https://doi.org/10.1373/jalm.2018.027409.
- Juttukonda, L.J.; Katz, S.; Gillon, J. Impact of a rapid blood culture diagnostic test in a children's hospital depends on Gram-positive versus Gram-negative organism and day versus night shift. J Clin Microbiol 2020, 58, e01400–e01419. https://doi.org/10.1128/JCM.01400-19.
- Bakhshi, K.; Mollaamin, F.; Monajjemi, M. Exchange and Correlation Effect of Hydrogen Chemisorption on Nano V(100) Surface: A DFT Study by Generalized Gradient Approximation (GGA). Journal of Computational and Theoretical Nanoscience 2011, 8, 763-768, https://doi.org/10.1166/jctn.2011.1750.
- Blauwkamp, T.A.; Thair, S.; Rosen, M.J. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat Microbiol 2019, 4, 663-674. https://doi.org/10.1038/s41564-018-0349-6.
- Hagen, A.; Eichinger, A.; Meyer-Buehn, M. Comparison of antibiotic and acyclovir usage before and after the implementation of an on-site FilmArray meningitis/encephalitis panel in an academic tertiary pediatric hospital: a retrospective observational study. BMC Pediatr 2020,20, 56. https://doi.org/10.1186/s12887-020-1944-2.
- Najaflou,N.; Monajjemi,M.; Attarikhasraghi,N.; Kandemirli,F.; Mollaamin,F. The Specification of Observed COVID-19 in England: A Review of Auto-Mutation. Biointerface Res. Appl. Chem2021, 11(6), 14794 – 14808. https://doi.org/10.33263/BRIAC116.1479414808.
- Lee, S.H.; Ruan, S.Y.; Pan, S.C.; Lee, T.F.; Chien, J.Y.; Hsueh P.R. Performance of a multiplex PCR pneumonia panel for the identification of respiratory pathogens and the main determinants of resistance from the lower respiratory tract specimens of adult patients in intensive care units. J Microbiol Immunol Infect 2019, 52, 920-928. https://doi.org/10.1016/j.jmii.2019.10.009.
- G. Guerriero,G. et al., Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes (Basel). 2018, 9(6) 34-46. https://doi: 10.3390/genes9060309.
- Yang, L.; Wen, K. S.; Ruan, X.; Zhao, Y.X.; Wei, F. and Wang, Q. Response of plant secondary metabolites to environmental factors, Molecules 2018, 23 (4), 1-26. https://doi: 10.3390/molecules23040762.
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: promising natural compounds against viral infections. Arch. Virol. 2017,162 (9), 2539-2551. https://doi: 10.1007/s00705-017-3417-y.
- Seema,T.M.;Thyagarajan,S.P. Pa-9: A flavonoid extracted from plectranthus amboinicus inhibits HIV-1 protease. Int. J. Pharmacogn. Phytochem. Res. 2016, 8(6), 1020-1024.
- Jo, S.; Kim, S.; Shin, D.H., Kim,M.S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzyme Inhib. Med. Chem. 2020, 35(1), 145-151, https://doi: 10.1080/14756366.2019.1690480.
- Akbulut, S. Medicinal Plants Preferences for the Treatment of COVID-19 Symptoms in Central and Eastern Anatolia, Kastamonu Univ., Journal of Forestry Faculty, 2021, 21(3): 196-207. https://doi:10.17475/kastorman.1048372.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; et al. Gaussian 09, Revision B.01. 2010. Gaussian Inc., Wallingford.
- Mollaamin, F.; Monajjemi, M. Harmonic Linear Combination and Normal Mode Analysis of Semiconductor Nanotubes Vibrations. J. Comput. Theor. Nanosci 2015, 12, 1030-1039. https://doi.org/10.1166/jctn.2015.3846.
- Roy, T. K.; Kopysov, V.; Pereverzev, A.; Šebek, J.; Gerber, R. B.; Boyarkin, O. V. Intrinsic structure of pentapeptide Leu-enkephalin geometry optimization and validation by comparison of VSCF-PT2 calculations with cold ion spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 24894–24901. https://doi.org/10.1039/c8cp03989e.
- Ni, W.; Li, G.; Zhao, J.; Cui, J.; Wang, R.; Gao, Z.; Liu, Y. Use of Monte Carlo simulation to evaluate the efficacy of tigecycline and minocycline for the treatment of pneumonia due to carbapenemase-producing Klebsiella pneumoniae. Infect Dis (Lond) 2018,50 ,507-513. https://doi.org/ 10.1080/23744235.2018.1423703.
- Kawczak, P.; Bober, L.; Bączek, T. QSAR analysis of selected antimicrobial structures belonging to nitro-derivatives of heterocyclic compounds. Lett Drug Des Discov 2018c, 17, 214-225 https://doi.org/10.2174/1570180815666181004112947.
- McArdle,S.;Mayorov,A.;Shan,X.;Benjamin,S.; Yuan,X. Digital quantum simulation of molecular vibrations. Chem. Sci. 2019, 10, 5725-5735. https://doi.org/10.1039/C9SC01313j.
- Wang, S. Efficiently Calculating Anharmonic Frequencies of Molecular Vibration by Molecular Dynamics Trajectory Analysis. ACS Omega 2019, 4, 9271- 9283. https://doi: 10.1021/acsomega.8b03364.
- Monajjemi, M.; Mollaamin, F.; Gholami, M.R.; Yoosbashizadeh, H.; Sadrnezhad, S.K.; Passdar H.Quantum chemical parameters of some organic corrosion inhibitors, pyridine, 2-picoline 4-picoline and 2,4-lutidine, adsorption at aluminum surface in hydrocholoric and nitric acids and comparison between two acidic media. MAIN GROUP MET CHEM 2003, 26, 349-361. https://doi.org/10.1515/MGMC.2003.26.6.349.
- Khaleghian, M.; Zahmatkesh, M.; Mollaamin, F.; Monajjemi, M. Investigation of Solvent Effects on Armchair Single-Walled Carbon Nanotubes: A QM/MD Study. Fuller. Nanotub. Carbon Nanostructures.,2011, 19, 251-261, https://doi.org/10.1080/15363831003721757.
- Lee, V.S.; Nimmanpipug, P.; Mollaamin, F.; Kungwan, N.; Thanasanvorakun, S.; Monajjemi, M. Investigation of single wall carbon nanotubes electrical properties and normal mode analysis: Dielectric effects. Russian Journal of Physical Chemistry A 2009, 83, 2288-2296, https://doi.org/10.1134/S0036024409130184.
- Beak P., Covington J.B., Smith S.G., White J.M., and Zeiger J.M.,1980. Displacement of protomeric equilibriums by self-association: hydroxypyridine-pyridone and mercaptopyridine-thiopyridone isomer pairs . J. Org. Chem 1980, 45, 1354-1362. https://doi.org/10.1021/jo01296a002.
- Sarasia, E.M.; Afsharnezhad, S.; Honarparvar, B.; Mollaamin, F.; Monajjemi, M. Estrogenic active stilbene derivatives as anti-cancer agents: A DFT and QSAR study. Phys. Chem. Liq. 2011, 49, 561-571. https://doi.org/10.1080/00319101003698992.
- Monajjemi, M.; Mahdavian, L.; Mollaamin, F.; Khaleghian, M. Interaction of Na, Mg, Al, Si with carbon nanotube (CNT): NMR and IR study. Russ. J. Inorg. Chem 2009, 54, 1465-1473. https://doi.org/10.1134/S0036023609090216.
- Polzella, M.S.; Lodeyro, P. Re-evaluating semi-empirical computer simulations in quantum chemistry. Found Chem 2019, 21, 83-95. https://doi.org/10.1007/s10698-018-09329-w.
- Kirkwood, J.G. On the Theory of Strong Electrolyte Solutions, J. Chem. Phys 1934, 2, 767. https://doi.org/10.1063/1.1749393.
- Kirkwood, J.G. The Dielectric Polarization of Polar Liquids. J. Chem. Phys 1939, 7, 911. https://doi.org/10.1063/1.1750343.
- Onsager, L. Electric Moments of Molecules in Liquids. J. Am. Chem. Soc 1936, 58, 1486-1493. https://doi.org/10.1021/ja01299a050.
- Wong, M.A.; Frisch, M.J.; Wiberg, K.B. Solvent effects.1.The mediation of electrostatic effects by solvents, J. Am. Chem. Soc 1991, 113, 4776-4782. https://doi.org/10.1021/ja00013a010.
- Zadeh, MAA.; Lari, H.; Kharghanian, L.; Balali, E.; Khadivi, R.; Yahyaei, H.; Mollaamin, F.; Monajjemi, M. Density Functional Theory Study and Anti-Cancer Properties of Shyshaq Plant: In View Point of Nano Biotechnology. J Comput Theor Nanosci 2015, 12, 4358-4367. https://doi.org/10.1166/jctn.2015.4366.
- Ghalandari, B.; Monajjemi, M.; Mollaamin, F. Theoretical Investigation of Carbon Nanotube Binding to DNA in View of Drug Delivery. J.Comput.Theor.Nanosci 2011, 8, 1212-1219, https://doi.org/10.1166/jctn.2011.1801.
- Monajjemi, M.; Farahani, N.; Mollaamin, F. Thermodynamic study of solvent effects on nanostructures: Phosphatidylserine and phosphatidylinositol membranes. Phys. Chem. Liq 2012, 50, 161-172. https://doi.org/10.1080/00319104.2010.527842.