- soils; Humus; Kaolinite; Atrazine; Trifuralin; Elovich model; sorption constant Kd; Freundlich model, persitance, leaching.
Copyright (c) 2022 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
ABSTRACT
Herbicides are one of the most widely used agrochemical classes around the world. They help farmers to protect their crops against weeds. However, they can move through the soil profile polluting water resources and adversely affect human health. Groundwater is an important source for the production of drinking water in many places of the world and the presence of pesticide residues in groundwater is a serious threat to the health of consumers of drinking water.
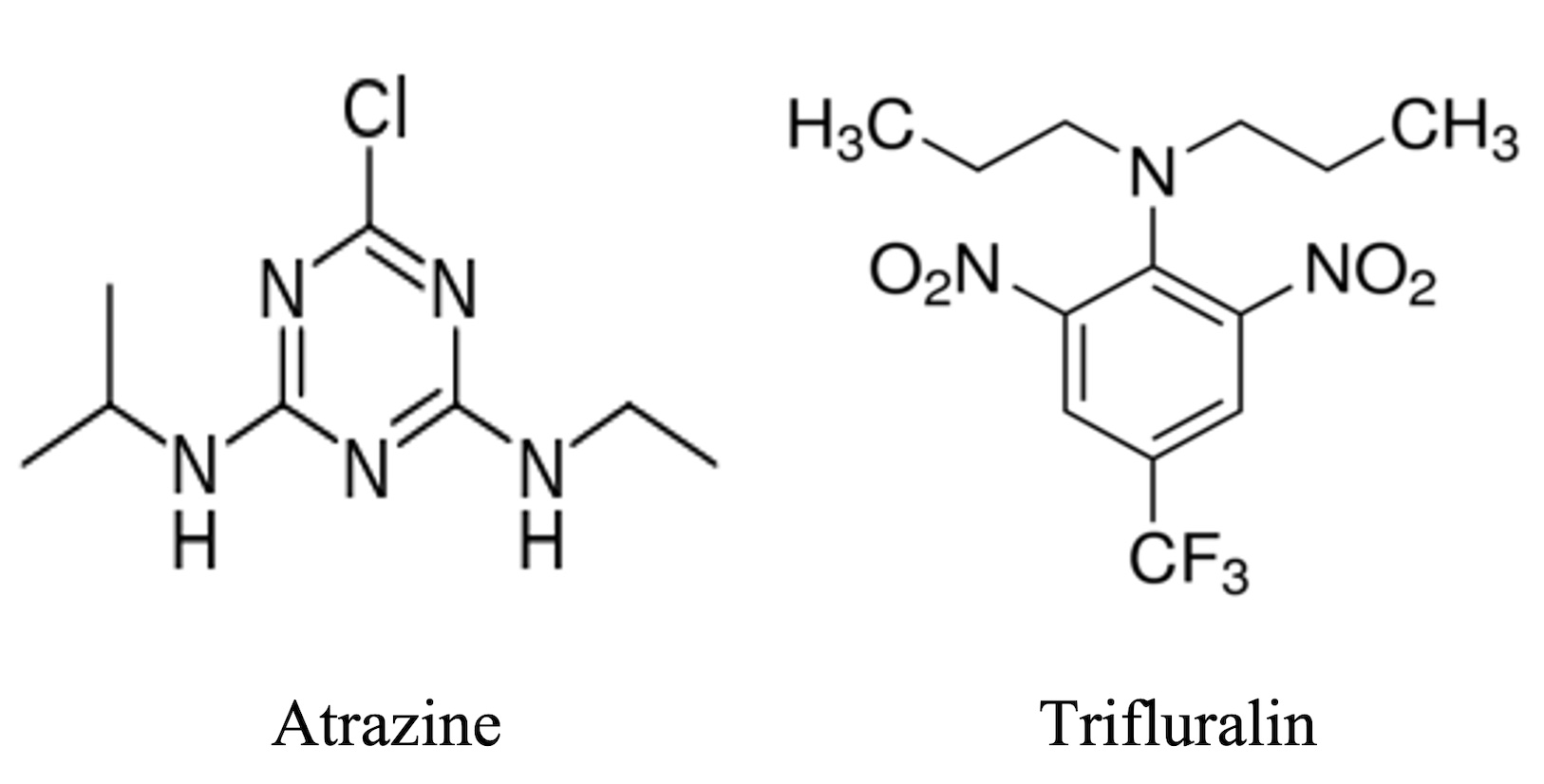
In this work, the behavior of two herbicides Atrazine and Trifluralin were study in an agricultural soil: Alhue soil and this soil modified whit clay (Kaolinite) and organic matter (Humus).
The original soil and modified soil samples were characterized by their physicochemical properties: pH, EC, OC and texture. The analytical method was optimized for the quantification of Atrazine and Trifluralin by High Performance Liquid Chromatography (HPLC). The contact time, adsorption/desorption isotherms, persistence of both compounds in the soil samples and modified soil samples with clay and/or organic matter was studied.
In general, all sorption curves for Trizine and Trifuralin in the modified soil samples were similar with relatively low adsorption for Trifuralin indicating that the soil modifications were not significant. The kinetic of sorption process was described by Elovich model
Both herbicides present a low Koc value, however, they present different types of adsorption, being for Atrazine a moderate adsorption and for Trifluralin a weak adsorption, which implies that both herbicides could be distributed in bodies of water as they are not fixed by organic matter. However, it should be noted that Atrazine presents higher Koc values than Trifluralin in all soil samples, which could mean that Atrazine would be less bioavailable than Trifluralin. Values obtained in the Pearson correlation of CO (%) and % of clay are expected since, when observing the results obtained in the Kd parameters for soils modified by both herbicides, they show that the higher the % of the physicochemical parameter, the higher the adsorption of the compound by the soil. The persistence of the pesticides showed that with both the addition of Kaolinite and Humus to the soil increases the half-life time for both herbicides. The results of the GUS index showed that both Atrazine and Trifluralin would be classified as leachable compounds in all soil samples
References
- V. REFERENCES
- F. A. Swartjes, Aa. M. Van der , Sci. Total Environ. 699, 134, (2020).
- R. P. Schwarzenbach, Science, 313, 1072, (2006).
- M. Sanchez, N. Perez, B. Quintanilla, Toxicol. Mech. Methods , 21, 681, (2011).
- P. L. Pingali, P. A. Roger, P.A. (Eds.). (1995).Impact of Pesticides on Farmer Health and the Rice Environment. Springer Science. 3-25.
- R. Loos, G. Locoro, S. Comero, S. Contini, D. Schwesig, F. Werres, P. Balsaa, O. Gans, S Weiss, L. Blaha, M. Bolchi, M. B. Gawlik, B.M., Water Res. 44, 4115, (2010).
- J. E. Barbash, G. P. Thelin, D. W. Kolpin, R. J. Gilliom, J. Environ. Qual. 30, 831, (2001).
- S. Navarro, N. Vela, G. Navarro, G., J. Agric. Res. 5, 357, (2007).
- N. Durñaes, L.A.B. Novo, C. Candeias, E. F. da Silva, 2018. Distribution, transport and fate of pollutants. In: Duarte, A.C., Cahada, A., Rocha-Santos, T. (Eds.), Soil Pollution: from Monitoring to Remediation. Academic Press, London, UK, pp. 29–56.
- J. Zolgharnein, A. Shahmoradi, J. Ghasemi, J., 2011. Pesticides removal using conventional and low-cost adsorbents: a review. Clean 39, 1105, (2011).
- S. Navarro, J. Hernàndez-Bastida, G. Cazaña, G. Pèrez-Lucas, J. Fenoll, J. Agric. Food Chem. 60, 5279, (2012).
- G. Briceño, G. Palma, N. Duràn, Environ. Sci. Technol. 37, 233, (2007).
- F. Sadegh-Zadeh, S. A. Wahid, B. Jalili, B., Environ. Technol. 2, 119, (2017).
- J. Fenoll, I. Garrido, P. Hellín, P. Flores, N. Vela, S. Navarro, Environ. Sci. Pollut. Res. 22, 4336, (2015).
- J. Fenoll, E. Ruiz, P. Flores, N. Vela, P. Hellín, S. Navarro, J. Hazard Mater. 187, 206, (2011).
- J. Fenoll, N. Vela, G. Navarro, G. Pérez-Lucas, S. Navarro, Sci. Total Environ. 493, 124, (2014).
- B. Gàmiz, R. Celis, L. Cox, M. C. Hermosín, J. Cornejo, Sci. Total Environ. 429, 292, (2012).
- J. M. Castillo, J. Beguet, F. Martin-Laurent, E. Romero, J. Hazard Mater. 304, 379, (2016).
- J. M. Martín-Benito, C. D. Brown, E. Herrero-Hernández, M. Arienzo, M. J. Sánchez- Martín, M. S. Rodríguez-Cruz, Sci. Total Environ. 589, 463, (2013).
- G. Pérez-Lucas, N. Vela, A. El Aatik, S. Navarro, 2019. Environmental risk of groundwater pollution by pesticide leaching through the soil profile. In: Larramendy, M.L., Soloneski, S. (Eds.), Pesticides. Use and Misuse and Their Impact in the Environment. IntechOpen, London, UK, pp. 45–71. .
- Organization for Economic Cooperation and Development (OECD), 2000. Guidelines for Testing of Chemicals, No 106, Adsorption-Desorption Using a Batch Equilibrium Method (Paris, France).
- A. E. A. Demir, F. B. Dilek, U. J. Yetis, Environ. Manag. 231, 1193, (2019).
- S. Baskaran, A. Rahmanb, R. W. Tillman, Pesticide Science. 46, 333, (1996).
- E. Barriuso, U. Baer, R. Calvet, J. Environ. Qual. 21, 359, (1992).
- Ellyn M. Murphy, John M. Zachara, Steven C. Smith, Jerry L.Phillips. Science of The Total Environment. 117–118, 413, (1992).
- G. J. Welhouse, W. F. Bleam, Environmental Science & Technology. 27, 594, (1993a).
- D.G. Schulze , Clay minerals, in: D Hillel (Ed.), Encyclopedia of Soils in the Environ- ment, Academic Press, New York, NY, 2005, pp. 246–254 .
- C. Scott, C. J. Jackson, C. W. Coppin, R. G. Mourant, M. E. Hilton, T. D. Sutherland, T.D.
- R. J. Russell, J. G. Oakeshott, J.G., 2009. Appl. Environ. Microbiol. 75, 2184, (2009).
- J. Liu, J., 2014. Atrazine. In: Wexler, P. (Ed.), Encyclopedia of Toxicology, third ed. Academic Press, pp. 336-338.
- A. H. Meyer, H. Penning, M. Elsner, M., Environ. Sci. Technol. 43, 8079, (2009).
- A. B. Baranda, A. Barranco, I. M. de Marañon, I.M., 2012. Water Res. 46, 669, (2012).
- G. Dinelli, C. Accinelli, A. Vicari, P. Catizone, J. Agric. Food Chem. 48, 3037, (2000).
- E. L. Kruger, L. Somasundaram, J. R. Coats, R. S. Kanwar, Environ. Toxicol. Chem. 12, 1959, (1993)..
- M. Qu, G. Liu, J. Zhao, H. Li, W. Liu, Y. Yan, X. Feng, D. Zhu, Environ. Pollut. 256, 113, (2020).
- P. K. Ghosh, L. Philip, L., Water Res. 38, 2277, (2004).
- J. L. Miller, A. G: Wollum J. B. Weber, J.B., J. Environ. Qual. 26, 633, (1997).
- T. C. C. Fernandes, M. A. Pizano, M. A. Marin-Morales, 2013. Characterization, modes of action and effects of trifluralin: a review. In: Price, A.J., Kelton, J.A. (Eds.), Herbicides-Current Research and Case Studies in Use. IntechOpen, UK, pp. 489-515.
- O. Tiryaki, K. Gozek, S. Khan, Bull. Environ. Contam. Toxicol. 59, 58, (1997).
- Sadzawka A, Carrasco MA, Grez R, Mora ML, Flores H, Neuman A. 2006. Métodos de Análisis de Suelos. Instituto de Investigaciones Agropecuarias (INIA). Serie Actas INIA 34, pag. 59–79.
- S. Venkatramanan, S. Chung, T. Ramkumar, G. Gnanachandrasamy, S. Vasudevan, Sci. Total Environ, 4, 109, (2013)
- A. M. Vangestel, Water, Air and Soil Pollution 88 (1-2), 119, (1996).
- R. Rodriguez, R. Linares, E. Guadalupe, E. (2009). Adsorción y desorción de cromo hexavalente en relaves mineros. Revista del Instituto de Investigaciones FIGMMG. 12 (Nº 24), 108-117. UNMSM.
- X. Domenech, (2000). QUIMICA DE SUELO: el impacto de los contaminantes. 3ra edición, Depto. De química de Barcelona. Cap. 4, 59-85.
- OECD. Guidelines for testing of chemicals, Section 1 (106):Adsorption-Desorption using batch equilibrium method in soils. Environmental Health and Safety Division, Organisation for Economic Co-operation and Development (OECD), Environment. Directorate, Paris, France. 2000
- X. Chen, Information 6, 14, (2015).
- M. R. Shariff, International Journal of Engineering Research and Development 1, 55, (2012).
- K. S. Ahmad, N. Rashid, M. F. Nazar, S. Tazaiyen, Journal of the Chemical Society of Pakistan 34-35, 1017, (2014).
- T. S. Arnarson, R. G. Keil, Org. Geochem. 32, 1401, (2001).
- B. K. G. Theng, Clay Miner. 23, 336, (1976).
- J. E. Peter, H. A. Sayari, Ind. Eng. Chem. Res. 45, 3248, (2006).
- W. J. Son, J. S. Choi, W. S. Ahn, Microporous Mesoporous Mater. 113, 31, (2008).
- A. F. Chamorro, R. D. Sanchez, Revista de Ciencias, Colombia 16, 145, (2012).
- V. Chantawong, N. Harvey, V. Bashkin, Water, Air and soil pollut. 148, 111, (2003).
- L. C. González–Márquez, A. M. Hansen, Rev. Mex. Cienc. Geol, 26, (2009)
- K. Müller, R. E. Smith, T. K. James, P. T. Holland, A. Rahman, Pest Manag. Sci. 59, 89, (2003).
- L. E. Nakagawa, L. C. Luchini, M. R. Musumeci, M. M. Andea, Pesq. Agrop. Bras.


