EVALUATION OF CYTOTOXIC EFFECT AGAINST TUMOUR CELLS OF THE ACIDIC POLYSACCHARIDES OF THE FUNGUS NOTHOPHELLINUS ANDINOPATAGONICUS
- acidic polysaccharides,
- fungi,
- Nothophellinus andinopatagonicus,
- anticarcinogenic activity,
- antioxidants
- cell cycle ...More
Copyright (c) 2022 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
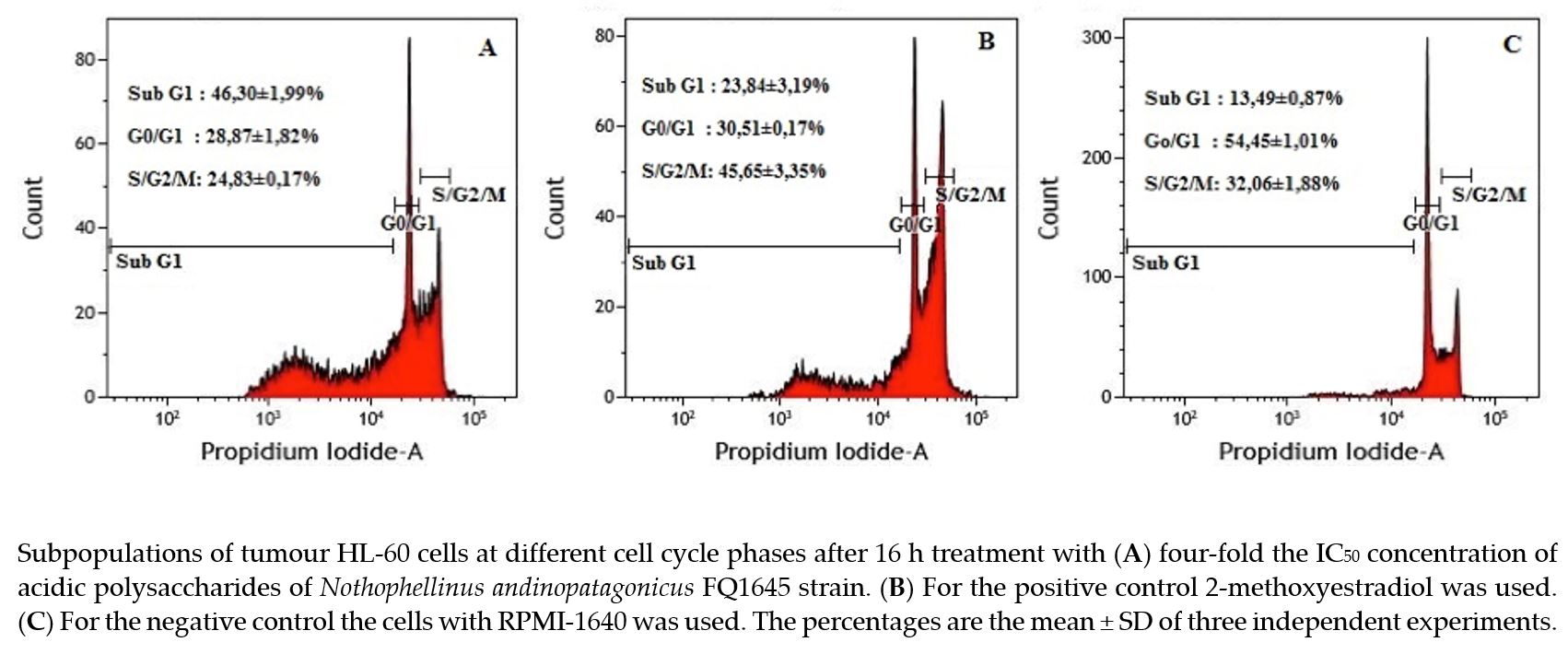
Fungal polysaccharides possess an important bioactive potential, including antioxidant and anticarcinogenic activity. The aim of this work was to determine the antioxidant activity and cytotoxicity against tumour and non-tumour cell lines acidic polysaccharides (NAAPs) of the fungus Nothophellinus andinopatagonicus. The effect of NAAPs on tumour cells lines was evaluated by MTT assay and flow cytometry. The analyses determined that glucose was the most abundant monomer and IR spectrum showed the typical peaks of β-glucans in the NAAPs. The cell viability assays revealed significant activity of NAAPs against HL-60, HCT-116 and MCF-7 tumour cell lines (IC50 = 767,16 µg mL-1, 1256 µg mL-1 and 4241,7 µg mL-1, respectively); but a much lower cytotoxicity against the non-tumour cell line HGF-1 (outside the range of the highest concentration tested (>10 mg mL-1)). NAAPs affected the cell cycle of HL-60 tumour cells, increasing the percentage of cells in the sub G1 phase and reducing it in the S/G2/M phases. Moreover, low concentrations of NAAPs also showed an effective cytotoxic activity against tumour cell lines while the non-tumour cell line was unaffected, maintaining a viability close to 100%. The antioxidant activity of the highest NAAPs concentration tested was 6.24% and 4.63%, for DPPH and ABTS method, respectively.
References
- Habtemariam S (2020) Trametes versicolor (Synn. Coriolus versicolor) polysaccharides in cancer therapy: Targets and efficacy. Biomedicines 8 (135): 1-26. Doi: 10.3390/biomedicines8050135
- Yan J.K, Pei J.J, Ma H.L, Wang Z.B, Liu Y.S (2017) Advances in antitumor polysaccharides from Phellinus sensu lato: Production, isolation, structure, antitumor activity, and mechanisms. Critical Reviews in Food Science and Nutrition 57 (6): 1256-1269. Doi: 10.1080/10408398.2014.984802
- Cid C, Herrera C, Rodíguez R, Bastías G, Jiménez J (2016) Assessing the economic impact of cancer in Chile: a direct and indirect cost measurement base on 2009 registries. Medwave 16 (7): 1-12. Doi: 10.5867/medwave.2016.07.6509
- Shalapour S, Karin M (2015) Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest 125 (9): 3347-3355. Doi: 10.1172/JCI80007
- Woo S.R, Corrales L, Gajewski T.F (2015) Innate immune recognition of cancer. Annu Rev Immunol 33: 445-474
- Pisoschi A.M, Pop A (2015) The role of antioxidants in the chemistry of oxidative stress: a review. European Journal of Medicinal Chemistry 97: 55-74. Doi: 10.1016/j.ejmech.2015.04.040
- Yan J.K, Wang Y.Y, Wang Z.B, Ma H.L, Pei J.J, Wu J.Y (2016) Structure and antioxidative property of a polysaccharide from an ammonium oxalate extract of Phellinus linteus. International Journal of Biological Macromolecules 91: 92-99. Doi: 10.1016/j.ijbiomac.2016.05.063
- Ruíz R, Strasser-Weippl K, Touya D, Herrero Vincent C, Hernández-Blanquisett A, St. Louis J, Bukowski A, Goss P.E (2017) Improving access to high-cost cancer drugs in Latin America: much to be done. Cáncer 123: 1313-1323. Doi: 10.1002/cncr.30549
- Kozarski M, Klaus A, Niksic M, Jakovlievic D, Helsper J.P.F.G, Griensven L.J.L.D (2011) Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chemistry 129: 1667-1675. Doi: 10.1016/j.foodchem.2011.06.029
- Giavasis I (2014) Bioactive fungal polysaccharides as potential functional ingredients in food and nutraceuticals. Current Opinion in Biotechnology 26: 162-173. Doi: 10.1016/j.copbio.2014.01.010
- Wang Z, Zhou F, Quan Y (2014) Antioxidant and immunological activity in vitro of polysaccharides from Phellinus nigricans mycelia. International Journal of Biological Macromolecules 64: 139-143. Doi: 10.1016/j.ijbiomac.2013.11.038
- Mei Y, Zhu H, Hu Q, Liu Y, Zhao S, Peng N, Liang Y (2015) A novel polysaccharide from mycelia of cultured Phellinus linteus displays antitumor activity through apoptosis. Carbohydrate Polymers 124: 90-97. Doi: 10.1016/j.carbpol.2015.02.009
- Yan J.K, Wang Y.Y, Ma H.L, Wang Z.B, Pei J.J (2016) Structural characteristics and antioxidant activity in vivo of a polysaccharide isolated from Phellinus linteus mycelia. Journal of the Taiwan Institute of Chemical Engineers 65: 110-117. Doi: 10.1016/j.jtice.2016.05.052
- Nakamura T, Matsugo S, Uzuka Y, Matsuo S, Kawagishi H (2004) Fractionation and anti-tumor activity of the mycelia of liquid-cultured Phellinus linteus. Biosci. Biotechnol. Biochem. 68 (4): 868-872. Doi: 10.1271/bbb.68.868
- Yan J.K, Wang Y.Y, Ma H.L, Wang Z.B (2016) Ultrasonic effects on the degradation kinetics, preliminary characterization and antioxidant activities of polysaccharides from Phellinus linteus mycelia. Ultrasonics Sonochemistry 29: 251-257. Doi: 10.1016/j.ultsonch.2015.10.005
- Doskocil I, Havlik J, Verlotta R, Tauchen J, Vesela L, Macakova K, Opletal L, Kokoska L, Rada V (2016) In vitro immunomodulatory activity, cytotoxicity and chemistry of some central European polypores. Pharmaceutical Biology 54 (11): 2369-2376. Doi: 10.3109/13880209.2016.1156708
- Lin C.J, Lien H.M, Lin H.J, Huang C.L, Kao M.C, Chen Y.A, Wang C.K, Chang H.Y, Chang Y.K, Wu H.S, Lai C.H (2016) Modulation of T cell response by Phelinus linteus. J. Biosci. Bioeng. 121: 84-88. Doi: 10.1016/j.jbiosc.2015.05.008
- Li S.C, Yang X.M, Ma H.L, Yan J.K, Gou D.Z (2015) Purification, characterization and antitumor activity of polysaccharides extracted from Phellinus igniarius mycelia. Carbohydrate Polymers 133: 24-30. Doi: 10.1016/j.carbpol.2015.07.013
- Wang Z.M, Peng X, Lee K.L.D, Tang J.C, Cheung P.C.K, Wu J.Y (2011) Structural characterization and immunomodulatory property of an acidic polysaccharide from mycelial culture of Cordyceps sinensis fungus Cs-HK1. Food Chemistry 125: 637-643. Doi: 10.1016/j.foodchem.2010.09.052
- Ukai S, Hirose K, Hiko T (1972) Isolations and characterizations of polysaccharides from Tremella fuciformis Berk. Chem Pharm Bull 20 (6): 1347-1348
- Ukai S, Hirose K, Hiko T, Hara C, Irikura T, Kanechika T, Hasegawa Y (1972) Antitumor activity on Sarcoma 180 of the polysaccharides from Tremella fuciformis Berk. Chem Pharm Bull 20 (10): 2293-2294
- Guo X, Zou X, Sun M (2010) Optimization of extraction process by response surface methodology and preliminary characterization of polysaccharides from Phellinus igniarius. Carbohydrates Polymers 80: 344-349. Doi: 10.1016/j.carbpol.2009.11.028
- Feng T, Cai J.L, Li X.M, Zhou Z.Y, Huang R, Zheng Y.S, Li Z.H, Liu J.K (2016) Phellibarin D with an unprecedented triterpenoid skeleton isolated from the mushroom Phellinus rhabarbarinus. Tetrahedron Letters 57: 3544-3546. Doi: 10.1016/j.tetlet.2016.06.114
- Liu M.M, Zeng P, Li X.T, Shi L.G (2016) Antitumor and immunomodulation activities of polysaccharide from Phellinus baumii. International Journal of Biological Macromolecules 91: 1199-1205. Doi: 10.1016/j.ijbiomac.2016.06.086
- Zhang Z.F, Lv G.Y, Song T.T, Jin Q.L, Huang J.B, Fan L.F, Cai W.M (2015) Comparison of the preliminary characterizations and antioxidant properties of polysaccharides obtained from Phellinus baumii growth on different culture substrates. Carbohydrates Polymers 132: 397-399. Doi: 10.1016/j.carbpol.2015.06.006
- Rajchenberg M (2006) Polypores (Basidiomycetes) from the Patagonian Andes Forests of Argentina; Bibliotheca Mycologica Band 201, J. Cramer Verlag: Stuttgart, Germany: pp. 203 - 206
- Rajchenberg M, Pildain M.B, Bianchinotti M.V, Barroetaveña C (2015) The phylogenetic position of poroid Hymenochaetaceae (Hymenochaetales, Basidiomycota) from Patagonia, Argentina. Mycologia 107 (4): 754-767. Doi: 10.3852/14-170
- Aqueveque P, Anke T, Saéz K, Silva M, Becerra J (2010) Antimicrobial activity of submerged cultures of Chilean Basidiomycetes. Planta Med 76: 1787-1791. Doi: 10.1055/s-0030-1249853
- Abdala-Díaz R.T, Casas Arrojo V, Arrojo Agudo M.A, Cárdenas C, Dobretsov S, Figueroa F.L (2019) Immunomodulatory and antioxidant activities of sulfated polysaccharides from Laminaria ochroleuca, Porphyra umbilicalis, and Gelidium corneum. Marine Biotechnology 21: 577-587. Doi: 10.1007/s10126-019-09905-x
- Figueroa F.A, Abdala-Díaz R.T, Hernández V, Pedreros P, Aranda M, Cabrera-Pardo J.R, Pérez C, Becerra J, Urrutia R (2019) Invasive diatom Didymosphenia geminata as a source of polysaccharides with antioxidant and immunomodulatory effects on macrophage cell lines. J Appl Phycol :1-10. Doi: 10.1007/s10811-019-01976-6
- Peredo K, Reyes H, Escobar D, Vega-Lara J, Berg A, Pereira M (2014) Acetylation of bleached Kraft pulp: Effect of xylan content on properties of acetylated compounds. Carbohydrate Polymers 117: 1014-1020. Doi: 10.1016/j.carbpol.2014.10.004
- Meng M, Cheng D, Han L, Chen Y, Wang C (2016) Isolation, purification, structural analysis and immunostimulatory activity of wáter-soluble polysaccharides from Grifola frondosa fruiting body. Carbohydrate polymers 157: 1134-1143. Doi: 0.1016/j.carbpol.2016.10.082
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55-63
- Afrin S, Giampieri F, Gasparrini M, Forbes-Hernández T.Y, Cianciosi D, Reboredo-Rodriguez P, Amici A, Quiles J.L, Battino M (2018) The inhibitory effect of Manuka honey on human colon cancer HCT-116 and LoVo cell growth. Part 1: the suppression of cell proliferation, promotion of apoptosis and arrest of the cell cycle. Food Funct 9: 2145-2157. Doi: 10.1039/c8fo00164b
- Brand-Williams W, Cuvelier M.E, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Lebensm-Wiss. u-Technol 28: 25-30
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine 26: 1231-1237
- Li T, Yang Y, Liu Y, Zhou S, Yan M.Q, Wu D, Zhang J, Tang C (2015) Physicochemical characteristics and biological activities of polysaccharide fractions from Phellinus baumii cultured with different methods. International Journal of Biological Macromolecules 81: 1082-1088. Doi: 10.1016/j.ijbiomac.2015.09.001
- Cao X, Liu R, Liu J, Huo Y, Yang W, Zeng M, Yang C (2013) A novel polysaccharide from Lentinus edodes mycelia exhibits potential antitumor activity on laryngeal squamous cancer cell lines Hep-2. Appl Biochem Biotechnol 171: 1444-1453. Doi: 10.1007/s12010-013-0441-6
- Su C.H, Lai M.N, Lin C.C, Ng L.T (2016) Comparative characterization of physicochemical properties and bioactivities of polysaccharides from selected medicinal mushrooms. Appl Microbiol Biotechnol 100: 4385-4393. Doi: 10.1007/s00253-015-7260-3
- Zhang M, Cheung P.C.K, Ooi V.E.C, Zhang L (2004) Evaluation of sulfated fungal β-glucan from the sclerotium of Pleurotus tuber-regium as a potential water-soluble anti-viral agent. Carbohydrate Research 339: 2297-2301. Doi: 10.1016/j.carres.2004.07.003
- Del Corno M, Gessani S, Conti L (2020) Shaping the innate immune response by dietary glucans: any role in the control of cancer?. Cancers 12 (155): 1-17. Doi: 10.3390/cancers12010155
- Cheng J.J, Chang C.C, Chao C.H, Lu M.K (2012) Characterization of fungal sulfated polysaccharides and their synergistic anticancer effects with doxorubicin. Carbohydrate Polymers 90: 134-139. Doi: 10.1016/j.carbpol.2012.05.005
- Ferreira I.C.F.R, Heleno S.A, Reis F, Stojkovic D, Queiroz M.J, Vasconcelos M.H, Sokovic M (2015) Chemical features of Ganoderma polysaccharides with antioxidant, antitumor and antimicrobial activities. Phytochemistry 114: 38-55. Doi: 10.1016/j.phytochem.2014.10.011
- Khan M, Zhang X, You L, Fu X, Abbasi A (2015) Chapter 59: Structure and bioactivities of fungal polysaccharides. In Polysaccharides; Ramawat, K.G., Mérillon, J.M.; Springer International Publishing, Switzerland, pp. 1851-1866. Doi: 10.1007/978-3-319-16298-0_28
- Meena M, Prasad V, Zehra A, Gupta V.K, Upadhyay R.S (2015) Mannitol metabolism during pathogenic fungal-host interactions under stressed conditions. Frontiers in Microbiology 6: 1-12. Doi: 10.3389/fmicb.2015.01019
- Wannet W.J.B, Hermans J.H.M, Van der Drift C, Op den Camp H.J.M (2000) HPLC detection of soluble carbohydrates involved in manitol and trehalose metabolism in the edible mushroom Agaricus bisporus. J Agric Food Chem 48: 287-291. Doi: 10.1021/jf990596d
- Kudo T, Takeuchi K, Ebina Y, Nakazawa M (2012) Inhibitory effects of trehalose on malignant melanoma cell growth: Implications for a novel topical anticancer agent on the ocular surface. ISRN Ophthalmology: 1-9.
- Allavena G, Del Bello B, Tini P, Volpi N, Valacchi G, Miracco C, Pirtoli L, Maellaro E (2018) Trehalose inhibits cell proliferation and amplifies long-term temozolomide- and radiation-induced cytotoxicity in melanoma cells: A role for autophagy and premature senescence. J Cell Physiol: 1-14. Doi: 10.1002/jcp.27838
- Huguet E.L, McMahon J.A, McMahon A.P, Bicknell R, Harris A.L (1994) Differential expression of human Wnt genes 2, 3, 4, and 7B in human breast cell lines and normal and disease states od human breast tissue. Cancer Research 54: 2615-2621.
- Akim A, Ling L.C, Rahmat A, Zakaria Z.A (2011) Antioxidant and anti-proliferative activities of Roselle juice on Caov-3, MCF-7, MDA-MB-231 and HeLa cancer cell lines. Afr J Pharm Pharmacol 5 (7): 957-965. Doi: 10.5897/AJPP11.207
- Roa I, Sánchez T, Majlis A, Schalper K (2012) Mutación del gen KRAS en el cáncer de colon y recto. Rev Med Chile 141: 1166-1172.
- Snarr B.D, Qureshi S.T, Sheppard D.C (2017) Immune recognition of fungal polysaccharides. J Fungi 3 (47): 1-29. Doi: 10.3390/jof3030047
- Llacuna L, Mach N (2012) Papel de los antioxidantes en la prevención del cáncer. Rev Esp Nutr Hum Diet 16 (1): 12-24
- Hao L, Sheng Z, Lu J, Tao R, Jia S (2016) Characterization and antioxidant activities of extracellular and intracellular polysaccharides from Fomitopsis pinicola. Carbohydrate Polymers 141: 54-59. Doi: 10.1016/j.carbpol.2015.11.048
- Wang J, Hu S, Nie S, Yu Q, Xie M (2016) Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxidative Medicine and Cellular Longevity: 1-12. Doi: 10.1155/2016/5692852