Vol 67 No 3 (2022): Journal of the Chilean Chemical Society
Original Research Papers
TARGETING THE MAIN PROTEASE AND THE SPIKE PROTEIN OF SARS-COV-2 WITH NATURALLY OCCURRING COMPOUNDS FROM SOME CAMEROONIAN MEDICINAL PLANTS: AN IN-SILICO STUDY FOR DRUG DESIGNING

Published
September 2, 2022
Keywords
- COVID-19,
- SARS-CoV-2,
- Main protease,
- Spike protein,
- Apiphytotherapy
- Molecular docking,
- Molecular dynamics,
- ADMET ...More
How to Cite
Fouedjou , R. T., Dongmo Fogang, H. P., Ouassaf, M., Qais, F. A., Bakhouch, M., Belaidi, S., & Chtita, S. (2022). TARGETING THE MAIN PROTEASE AND THE SPIKE PROTEIN OF SARS-COV-2 WITH NATURALLY OCCURRING COMPOUNDS FROM SOME CAMEROONIAN MEDICINAL PLANTS: AN IN-SILICO STUDY FOR DRUG DESIGNING. Journal of the Chilean Chemical Society, 67(3), 5602-5614. Retrieved from https://jcchems.com/index.php/JCCHEMS/article/view/1996
Copyright (c) 2022 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
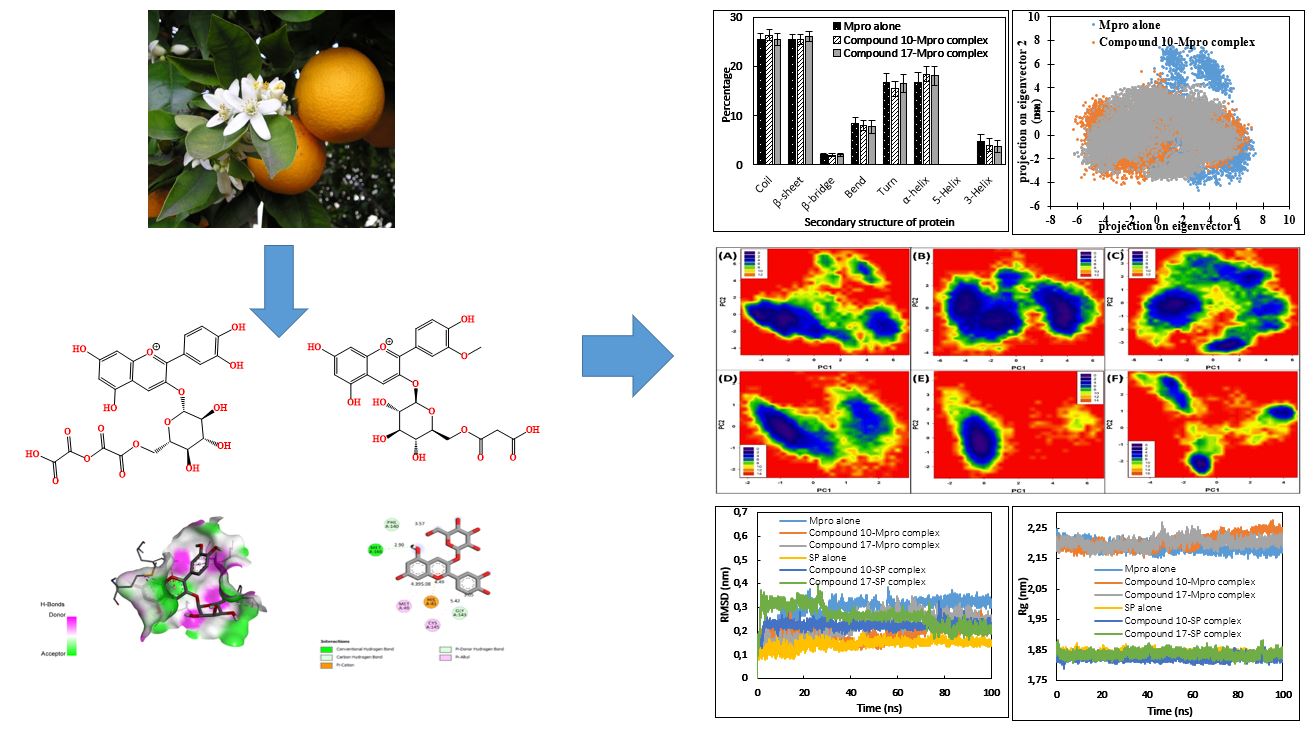
Despite the social distancing and hygiene rules prescribed by the WHO, the novel Corona-virus is still on the way of a significant rapid rise in deaths. Therefore, identification of chemotherapeutic drugs against Corona Viral Infection all around the world is still requires. Some medicinal plants have a valuable therapeutic effect when mixt with honey, the obtained formulations are preliminary use in Cameroon against viral infection particularly respiratory infections. In this work, we looked for the potential anti-SARS-CoV-2 molecule throw execution of in silico computational studies of six Cameroonian plants intervening in the treatment respiratory infections in apiphytotherapy. AutoDock Vina was used for docking studies against SARS-CoV-2 Mpro and SP. We further conducted of pharmacokinetics properties and the safety profile of compounds with the top score in order to identify the best drug candidates. Totally 100 compounds were screened, of these, eighteen showed high binding affinity against SARS-CoV-2 Mpro and SP. The results suggest the effectiveness of compounds 10 and 17 obtained from Citrus Sinensis as potent drugs against SARS-CoV-2 as they tightly bind to its Mpro and SP with low binding energies. The stability of the two compounds complexed with Mpro and SP was validated through MD simulation. The availability of potent protein inhibitors and diverse of compounds from Cameroon flora scaffolds indicate the feasibility of developing potent main protease and spikes proteins inhibitors as antivirals for COVID-19. Based on further in vivo and in vitro experiments and clinical trials, some of these phytoconstituents could be proposed for effective inhibition of the replication of the SARS-CoV-2.References
- Alavijeh, M. S., Chishty, M., Qaiser, M. Z. & Palmer A. M. (2005). Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. The Journal of the American Society for Experimental NeuroTherapeutics, 4, 554-571. https://doi.org/10.1602/neurorx.2.4.554
- Araya, J. J., Zhang, H., Prisinzano, T. E., Mitscher, L. A. & Timmermann, B. N. (2011). Identification of unprecedented purine-containing compounds, the zingerines, from ginger rhizomes (Zingiber officinale Roscoe) using a phase-trafficking approach. Phytochemistry, 72, 935-941. http://doi.org/10.1016/j.phytochem.2011.03.007
- Awortwe, C., Fasinu, S. P. & Rosenkranz, B. (2014). Awortwe C, Fasinu PS, Rosenkranz B. Application of Caco-2 cell line in herb-drug interaction studies: current approaches and challenges. Journal of Pharmacy & Pharmaceutical Sciences, 17, 1-19. http://doi.org/10.18433/j30k63
- Bassoléa, I. H. N., Lamien-Meda, A., Bayala, B., Obame, L. C., Ilboudo, A. J., Franz, C., Novak, J., Nebié, R. C. & Dicko, M.H. (2011). Chemical composition and antimicrobial activity of Cymbopogon citratus and Cymbopogon giganteus essential oils alone and in combination. Phytomedicine 18, 1070-1074. https://doi.org/10.1016/j.phymed.2011.05.009
- Berendsen, H., Van Der Spoel, D., & Van Drunen, R. (1995). GROMACS: Amessage-passing parallel molecular dynamics implementation. Computer Physics Communications, 91(1–3), 43-56. https://doi.org/10.1016/0010-4655(95)00042-E
- Bussi, G., Donadio, D., & Parrinello, M. (2007). Canonical sampling through velocity rescaling. Journal of Chemical Physics, 126(1), 014101. https://doi.org/10.1063/1.2408420
- Chirila P. (1987). Medicinănaturistă. Mic tratatterapeutic, Ed. Medicală, Bucureşti, p 285-547. https://doi.org/10.1007/978-94-017-9199-1_15
- Chtita, S., Belhassan, A., Aouidate, A., Belaidi, S., Bouachrine, M. & Lakhlifi, T. (2021). Discovery of potent SARS-CoV-2 inhibitors from approved antiviral drugs via docking and virtual screening. Combinatorial Chemistry & High Throughput Screening, 24(3), 441-454. https://doi.org/10.2174/1386207323999200730205447
- Coelho, C. M. M., Bellato, C. de M., Santos, J. C. P., Ortega, E. M. M., & Tsai, S. M. (2007). Effect of phytate and storage conditions on the development of the "hard-to-cook". Journal of the Science of Food and Agriculture, 1243, 1237-1243. https://doi.org/10.1002/jsfa
- Dassault Systèmes, BIOVIA, Discovery Studio Visualizer, San Diego: Dassault Systèmes BIOVIA (2020). Available from: http://www. 3dsbiovia.com/products/collaborative-science/biovia-discovery-studio/visualization-download.php
- Domingo, E. (2020). Molecular basis of genetic variation of viruses. Virus as Populations 35-71. https://doi.org/10.1016/B978-0-12-816331-3.00002-7
- Dorato, M. A., & Buckley, L. A. (2007). Toxicology testing in drug discovery and development, current protocols in toxicology. https://doi.org/10.1002/0471141755.tx1901s31
- Fan, J., & de Lannoy I. A. M. (2014). Pharmacokinetics, Review. Biochemical Pharmacology, 87, 93-120. https://doi.org/10.1016/j.bcp.2013.09.007
- Finch, A. & Pillans, P. (2014). P-glycoprotein and its role in drug-drug interactions. Australian Prescriber, 37(4), 137-139. https://doi.org/10.18773/austprescr.2014.050
- Fotso, K. P. R., Meutchieye, F., Andriamanalina, S. I., Youbissi, A., Tchoumboué, J., Pinta, J. Y. & Zango, P. (2014). Caractéristiques socio-économiques et techniques de l’apiculture dans les Départements de Bamboutos, Mifi et Menoua (Région de l’Ouest-Cameroun). Livestock Research for Rural Development. 26(221). http://www.lrrd.org/lrrd26/12/fots26221.htm
- Fouedjou, T. R., Chtita, S., Bakhouch, M., Belaidi, S., Ouassaf, M., Djoumbissie, A. L., Tapondjou, A. L. & Qais, A. F. (2021). Cameroonian Medicinal plants as Potential candidates of SARS-CoV-2 Inhibit. Journal of Biomolecular Structure and Dynamics. https://doi.org/10.1002/jps.20536
- Fujita, T., Urban, J. T., Leabman, K. M., Fujita, K. & Giacomini, M. K. (2006). Transport of drugs in the kidney by the human organic cation transporter, OCT2 and its genetic variants. Journal of Pharmaceutic al Sciences, 95(1), 25-36. https://doi.org/10.1080/07391102.2020.1778535
- Hilgendorf, C., Ahlin, G., Seithel, A, Artursson, P, Ungell, AL, Karlsson, J. (2007). Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic Cell Lines. Drug Metabolism and Disposition, 35(8), 1333-1340. http://doi.org/10.1124/dmd.107.014902
- Hudaib, M. & Aburja, T. (2007). Volatile components of Thymus vulgaris L. from wild-growing and cultivated plants in Jordan. Flavour and Fragrance Journal, 22, 322-327. https://doi: 10.1002/ffj.1800
- Hudaib, M., Speroni, E., Di Pietra, A. M., & Cavrini, V. (2002). GC/MS evaluation of thyme (Thymus vulgaris L.) oil composition and variations during the vegetative cycle. Journal of Pharmaceutical and Biomedical Analysis, 29(4), 691-700. https://doi.org/10.1016/S0731-7085(02)00119-X
- Kelebek, H., Canbas, H. & Selli, S., 2008. Determination of phenolic composition and antioxidant capacity of blood orange juices obtained from cvs. Moro and Sanguinello (Citrus sinensis (L.) Osbeck) grown in Turkey. Food Chemistry 107, 1710-1716. https://doi.org/10.1016/j.foodchem.2007.10.004
- Kikuzaki, H., Tsai, S.-M. & Nakatanit, N., (1992). Gingerdiol Related Compounds from the rhizomes of Zingiber officinale. Phytochemistry, 31(5), 1783-1786. https://doi.org/10.1016/0031-9422(92)83147-Q
- König, J., Müller, F. & Fromm, F. M. (2013). Transporters and Drug-Drug Interactions: Important Determinants of Drug Disposition and Effects. Pharmacological Reviews 65:944-966. https://doi.org/10.1124/pr.113.007518
- Kumari, R., Kumar, R. & Lynn, A. (2014). g_mmpbsa —A GROMACS Tool for High-Throughput MM-PBSA Calculations. Journal of Chemical Information and Modeling, 54(7), 1951–1962. https://doi.org/10.1021/ci500020m
- Lee, S. W., Lim, J. H., Kim, S. M., Jeong, J. H., Song, G. Y., Lee, S. W. & Rho, C. M. (2011). Phenolic compounds isolated from Zingiber officinale roots inhibit cell adhesion. Food Chemistry, 128(3), 778-782. https://doi.org/10.1016/j.foodchem.2011.03.095
- Li, F. (2016). Structure, Function, and Evolution of Coronavirus SpikeProteins. Annual Review of Virology 3, 237-61. https://doi.org/10.1146/annurev-virology-110615-042301
- Li, F., Li, W., Farzan, M. & Harrison, S.C. (2005). Structure of SARS corona virus spike receptor-binding domain complexed with receptor. Science, 309(5742), 1864-1868. https://doi.org/10.1126/science.1116480
- Li, R., Chen, W.-C., Wang, W.-P., Wen-yan Tian, W.-Y. & Zhang, X.-G. (2010). Extraction of essential oils from garlic (Allium sativum) using ligarine as solvent and its immunity activity in gastric cancer rat. Medicinal and Chemical Research, 19:1092-110. https://doi:10.1007/s00044-009-9255-z
- Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney P. J. (2001). Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced Drug Delivery Reviews, 1(46), 3-26. https://doi.org/10.1016/s0169-409x(00)00129-0
- Lu, H. (2020). Drug treatment options for the 2019-new coronavirus (2019-nCoV). Bioscience Trends, 16;14(1):69-71. http://doi;org/10.5582/bst.2020.01020
- Mahanta, J. J., Chutia, M., Bordoloi, M., Pathak, M. G., Adhikary, R. K. & Sarma, T. C. (2007). Cymbopogon citratus L. essential oil as a potential antifungal agent against key weed moulds of Pleurotus spp. Spawns. Flavour and Fragrance Journal, 22, 525-530. https://doi.org/10.1002/ffj.1835
- Maier, J. A., Martinez, C., Kasavajhala, K., Wickstrom, L., Hauser, K. E. & Simmerling, C. (2015). ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. Journal of Chemical Theory and Computation, 11(8), 3696–3713. https://doi.org/10.1021/acs.jctc.5b00255
- Marino, A. M., Yarde, M., Patel, H., Chong, S. & Balimane, P.V. (2005). Validation of the 96 well Caco-2 cell culture model for high throughput permeability assessment of discovery compounds. International Journal of Phamaceutics, 297(1-2), 235-241. http://doi.org.10.1016/j.ijpharm.2005.03.008
- Minovski, N., Andrej, P. & Tom, S. (2012). « Combinatorially-Generated Library of 6-Fluoroquinolone Analogs as Potential Novel Antitubercular Agents: A Chemometric and Molecular Modeling Assessment ». Journal of Molecular Modeling, 18(5), 1735 1753. http://doi.org.10.1007/s00894-011-1179-0
- Mitiku, S. B., Sawamura,¬ M., Itoh, T. & Ukeda, H. (2000). Volatile components of peel cold-pressed oils of two cultivars of sweet orange (Citrus sinensis (L.) Osbeck) from Ethiopia. Flavour and Fragrance Journal, 15, 240-244. https://doi.org/10.1002/1099-1026(200007/08)15:4<240::AID-FFJ902>3.0.CO;2-F
- Mogren, M. L., Olsson, E. M. & Gertsson, E. U. (2007). Quercetin content in stored onions (Allium cepa L.): effects of storage conditions, cultivar, lifting time and nitrogen fertiliser level. Journal of the Science of Food and Agriculture, 87, 1598-1602. https://doi.org/10.1002/jsfa.2904
- Morris, G. M., Huey, R., Lindstrom, W., Sanner, M. F., Belew, R. K., Goodsell, D. S. & Olson, A. J. (2009). AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. Journal of Computational Chemistry, 30(16), 2785-2791. http://doi.org.10.1002/jcc.21256
- Narkhede, R. R., Cheke, S. R., Ambhore, J. P. & Shinde, S. D. (2020). The Molecular Docking Study of Potential Drug CandidatesShowing Anti-COVID-19 Activity by Exploring of TherapeuticTargets of SARS-CoV-2. Eurasian Journal of Medicine and Oncology, 4(3):185-195. http://doi.org.10.14744/ejmo.2020.31503
- Nnomo, R. D., Tchouamo, I. R. & Pinta, J. Y. (2009). Apiphytothérapie à base du miel au Cameroun. Ethnopharmacologia, 44, 56-63.
- Oie, S. (1986). Drug distribution and binding. Journal of Clinical Pharmacology, 26(8):583-586. http://doi:10.1002/j.1552-4604.1986.tb02953.x
- Parrinello, M., & Rahman, A. (1981). Polymorphic transitions in single crystals: A new molecular dynamics method. Journal of Applied Physics, 52(12), 7182–7190. https://doi.org/10.1063/1.328693
- Pillaiyar, T., Meenakshisundaram, S. & Manickam, M. (2020). Recent discovery and development of inhibitors targeting coronaviruses. Drug Discovery Today. 25(4), 668-688. http://doi:10.1016/j.drudis.2020.01.015
- Pires, D.E.V., Blundell, T.L. & Ascher, D.B. (2015). pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures, J Med Chem. 58(9):4066-4072. https://doi.org/10.1021/acs.jmedchem.5b00104
- Prakash, D., Singh, N. B. & Upadhyay, G. (2007). Antioxidant and free radical scavenging activities of phenols from onion (Allium cepa). Food Chemistry 102, 1389-1393. https://doi.org/10.1016/j.foodchem.2006.06.063
- Qais, F. A., Sarwar, T., Ahmad, I., Khan, R. A., Shahzad, S. A. & Husain, F. M. (2021). Glyburide inhibits non-enzymatic glycation of HSA: An approach for the management of AGEs associated diabetic complications. International Journal of Biological Macromolecules, 169, 143–152. https://doi.org/10.1016/j.ijbiomac.2020.12.096.
- Rath, B., Abul Qais, F., Patro, R., Mohapatra, S. & Sharma, T. (2021). Design, synthesis and molecular modeling studies of novel mesalamine linked coumarin for treatment of inflammatory bowel disease. Bioorganic & Medicinal Chemistry Letters, 128029. https://doi.org/10.1016/j.bmcl.2021.128029
- Razzaghi-Abyaneh, M., Shams-Ghahfarokhi, M., Rezaee, M.-B., Jaimand, K., Alinezhad, S., R. & Yoshinari, T. (2009). Chemical composition and antiaflatoxigenic activity of Carum carvi L., Thymus vulgaris and Citrus aurantifolia essential oils. Food Control 20, 1018-1024. https://doi:10.1016/j.foodcont.2008.12.007
- Rota, M. C., Herrera, A., Martínez, R. M., Sotomayor, J. A., & Jordán, M. J. (2008). Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control, 19(7), 681-687. https://doi.org/10.1016/j.foodcont.2007.07.007
- Schoeman, D. & Fielding, C.B. (2019). Coronavirus envelope protein: current knowledge. Virology Journal, 16(69), 1-22. https://doi.org/10.1186/s12985-019-1182-0
- Selli, S. & Kelebek, H. (2001). Aromatic profile and odour-activity value of blood orange juices obtained from Moro and Sanguinello (Citrus sinensis L. Osbeck). Industrial Crops and Products, 33, 727-733. http://doi:10.1016/j.indcrop.2011.01.016
- Sepúlveda-Arias, J. C., Veloza, L. A., Escobar, L. M., Orozco, L. M. & Lopera, I.A. (2013). Anti-inflammatory effects of the main constituents and epoxides derived from the essential oils obtained from Tagetes lucida, Cymbopogon citratus, Lippia alba and Eucalyptus citriodora. The Journal of Essential Oil Research, 1-8. http://dx.doi.org/10.1080/10412905.2012.751556
- Siddiqui, S., Ameen, F., Jahan, I., Nayeem, S. M. & Tabish, M. (2019). A comprehensive spectroscopic and computational investigation on the binding of the anti-asthmatic drug triamcinolone with serum albumin. New Journal of Chemistry, 43(10), 4137-4151. https://doi.org/10.1039/C8NJ05486J
- Siddiqui, S., Ameen, F., Kausar, T., Nayeem, S. M., Ur Rehman, S. & Tabish, M. (2021). Biophysical insight into the binding mechanism of doxofylline to bovine serum albumin: An in vitro and in silico approach. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 249, 119296. https://doi.org/10.1016/j.saa.2020.119296
- Sofowora A. (1996). Plantes médicinales et médecine traditionnelle d’Afrique, Paris, Ed Karthala, 375 p.
- Sousa Da Silva, A. W. & Vranken, W. F. (2012). ACPYPE - AnteChamber PYthon Parser interfacE. BMC Research Notes. https://doi.org/10.1186/1756-0500-5-367
- Tchoumboué, J., Tchouamo, I. R., Pinta, J. Y. & Njia, M. N. (2001). Caractéristiques socioéconomiques et techniques de l’apiculture dans les Hautes Terres de l’Ouest du Cameroun, Tropicultura, 19(3) 141-146.
- Toutain, P.L. & Bousquet-Mélou, A (2004). Volumes of distribution. Journal of Veterinary Pharmacology and Therapeutics, 27(6):441-53. http://doi.org/10.1111/j.1365-2885.2004.00602.x
- Trott, O. & Olson, A. J. (2010). AutoDockVina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry, 31(2), 455-461. http://doi.org/10.1002/jcc.21334
- Vagen, M. I. & Slimestad, R. (2008). Amount of characteristic compounds in 15 cultivars of onion (Allium cepa L.) in controlled field trials. Journal of the Science of Food and Agriculture, 88, 404-411. https://doi.org/10.1002/jsfa.3100
- Van Der Spoel, D., Lindahl, E., Hess, B., Groenhof, G., Mark, A. E. & Berendsen, H. J. C. (2005). GROMACS: Fast, flexible, and free. Journal of Computational Chemistry, 26(16), 1701–1718. https://doi.org/10.1002/jcc.20291
- Wang, H., X. & NG, B. T. (2004). Isolation of Allicepin, a Novel Antifungal Peptide from Onion (Allium cepa) Bulbs. Journal of Peptide Science, 10, 173-177. https://doi:10.1002/psc.509
- WHO: Geneva, Switzerland. Coronavirus disease 2019 (COVID-19) Situation Report-38. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200227-sitrep-38-covid-19.pdf?sfvrsn=9f98940c_2 (Accessed on 28th May 2021). 2020.
- Yuan, L., Ji, F. T., Wang, G. A., Yang, B. J., & Su, L. Y. (2007). Two new furostanol saponins from the seeds of Allium cepa L. Chinese Chemical Letters, 19, 461-464. https://doi:10.1016/j.cclet.2008.02.005