NEW CHITOSAN-BASED CHEMO PHARMACEUTICAL DELIVERY SYSTEMS FOR TUMOR CANCER TREATMENT: SHORT-REVIEW

- Tumor cancer,
- Chemotherapy agents,
- Drug delivery,
- Chitosan,
- Bioavailability
Copyright (c) 2022 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
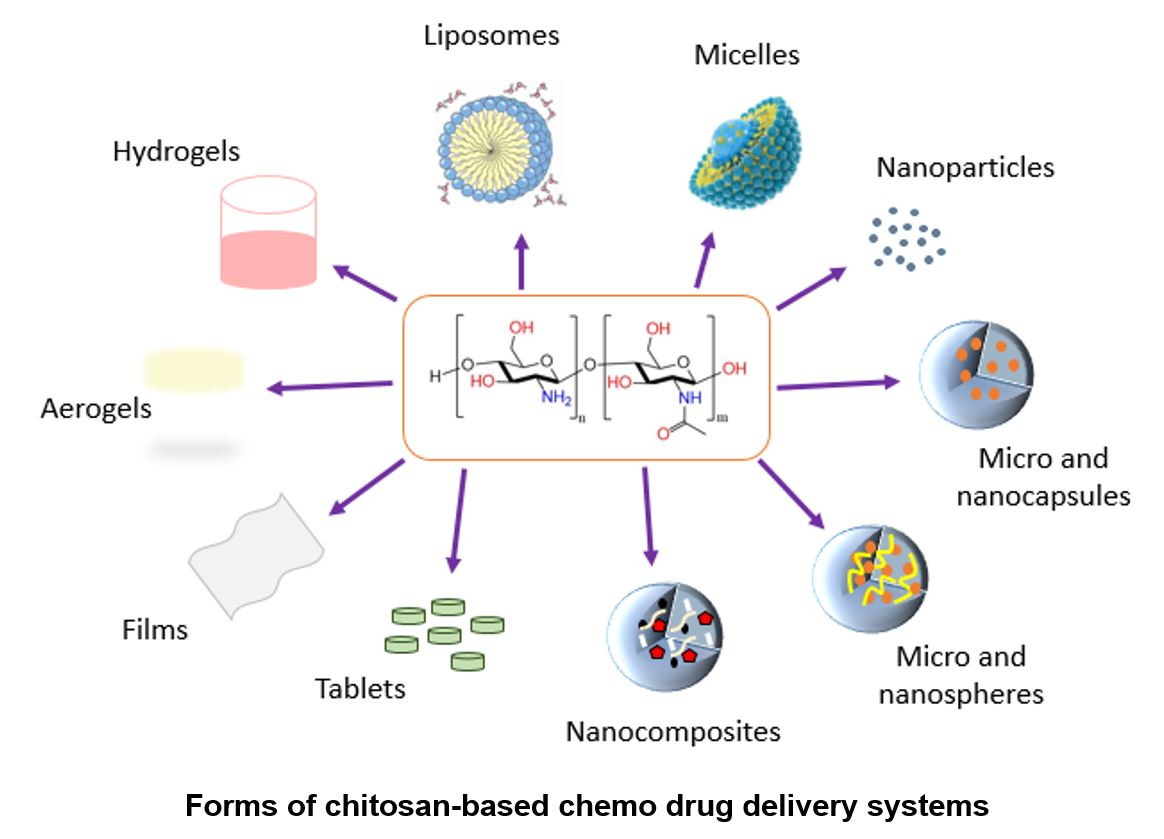
The aim of this review is to provide an overview of the delivery systems of chitosan-based chemotherapy agents that have been developed for the treatment of tumor cancer. Cancer treatment is a challenge that has always provided opportunities in different areas of study, due to its very complexity. Innovative options to make chemotherapy an effective treatment by targeting drugs to cancer cells through different modifications in delivery systems are being investigated. Chitosan, a biopolymer that is obtained from the partial deacetylation of chitin (the second most abundant biopolymer on earth) and is present in the exoskeleton of crustaceans, some insects, and also in the cell wall of some fungi. Chitosan has specific characteristics of solubility, functional groups in its structure, crosslinking power, affinity with other materials, biocompatibility, biodegradability, muco adhesiveness, provides bioavailability of the chemotherapeutic agent on cancer cells, without harming healthy cells. This document compiles some interesting studies on the use of chitosan in conjugation with other agents and safe materials for use in biomedicine, for the design, characterization, and development of new transport systems for chemotherapeutic agents, increasing the efficacy of this therapy in cancer treatment tumors.
References
- REFERENCES
- OMS, “Cáncer,” Organización Mundial de la Salud, 2018. [Online]. Available: https://www.who.int/es/news-room/fact-sheets/detail/cancer. [Accessed: 10-Oct-2019].
- International Agency for Research on Cancer, “Cancer Tomorrow,” Global Cancer Observatory, 2018. [Online]. Available: https://gco.iarc.fr/tomorrow/graphic-isotype?type=0&population=900&mode=population&sex=0&cancer=39&age_group=value&apc_male=0&apc_female=0. [Accessed: 10-Oct-2019].
- N. C. Institute, “A to Z List of Cancer Types - National Cancer Institute.” [Online]. Available: https://www.cancer.gov/types. [Accessed: 13-Sep-2021].
- E. S. dos Santos, V. P. Wagner, J. Cabral Ramos, D. W. Lambert, R. M. Castilho, and A. F. Paes Leme, “Epigenetic modulation of the tumor microenvironment in head and neck cancer: Challenges and opportunities,” Crit. Rev. Oncol. Hematol., vol. 164, no. May, p. 103397, 2021.
- D. Ribatti, T. Annese, and R. Tamma, “Controversial role of mast cells in breast cancer tumor progression and angiogenesis,” Clin. Breast Cancer, 2021.
- Y. Deng, S. Y. Liu, S. L. Chua, and B. L. Khoo, “The effects of biofilms on tumor progression in a 3D cancer-biofilm microfluidic model,” Biosens. Bioelectron., vol. 180, no. February, p. 113113, 2021.
- S. Mukhopadhyay, K. K. Mahapatra, P. P. Praharaj, S. Patil, and S. K. Bhutia, “Recent progress of autophagy signaling in the tumor microenvironment and its targeting for possible cancer therapeutics,” Semin. Cancer Biol., 2021.
- Q. Hu and Y. Luo, “Recent advances of polysaccharide-based nanoparticles for oral insulin delivery,” Int. J. Biol. Macromol., vol. 120, pp. 775–782, 2018.
- Cancer.Net, “Understanding Chemotherapy | Cancer.Net,” 2019. [Online]. Available: https://www.cancer.net/navigating-cancer-care/how-cancer-treated/chemotherapy/understanding-chemotherapy. [Accessed: 14-Sep-2021].
- Z. Mármol, G. Páez, M. Rincón, K. Araujo, and C. Aiello, “Quitina y Quitosano polímeros amigables . Una revisión de sus aplicaciones Chitin and Chitosan friendly polymer . A review of their applications,” Rev. Tecnocientifica URU, no. August 2016, pp. 53–58, 2011.
- M. T. Taghizadeh, H. Ashassi-Sorkhabi, R. Afkari, and A. Kazempour, “Cross-linked chitosan in nano and bead scales as drug carriers for betamethasone and tetracycline,” Int. J. Biol. Macromol., vol. 131, pp. 581–588, 2019.
- S. Naskar, S. Sharma, and K. Kuotsu, “Chitosan-based nanoparticles: An overview of biomedical applications and its preparation,” J. Drug Deliv. Sci. Technol., vol. 49, no. June 2018, pp. 66–81, 2019.
- S. Rodrigues, M. Dionísio, C. R. López, and A. Grenha, “Biocompatibility of Chitosan Carriers with Application in Drug Delivery,” J. Funct. Biomater., vol. 3, no. 3, pp. 615–641, 2012.
- E. M. Costa, S. Silva, M. Veiga, P. Baptista, F. K. Tavaria, and M. E. Pintado, “Textile dyes loaded chitosan nanoparticles: Characterization, biocompatibility and staining capacity,” Carbohydr. Polym., vol. 251, no. August 2020, p. 117120, 2021.
- B. Lima Santos Klienchen Dalari, C. Lisboa Giroletti, L. Dalri-Cecato, D. Gonzaga Domingos, and M. E. Nagel Hassemer, “Application of heterogeneous photo-Fenton process using chitosan beads for textile wastewater treatment,” J. Environ. Chem. Eng., vol. 8, no. 4, p. 103893, 2020.
- S. Sady, A. Błaszczyk, W. Kozak, P. Boryło, and M. Szindler, “Quality assessment of innovative chitosan-based biopolymers for edible food packaging applications,” Food Packaging Shelf Life, vol. 30, no. September 2021.
- Z. Wu, S. Tang, W. Deng, J. Luo, and X. Wang, “Antibacterial chitosan composite films with food-inspired carbon spheres immobilized AgNPs,” Food Chem., vol. 363, no. June, p. 130342, 2021.
- H. Park et al., “Flexible and disposable paper-based gas sensor using reduced graphene oxide/chitosan composite,” J. Mater. Sci. Technol., vol. 101, no. 2, pp. 165–172, 2022.
- T. Ahmed et al., “Bioengineered chitosan-magnesium nanocomposite: A novel agricultural antimicrobial agent against Acidovorax oryzae and Rhizoctonia solani for sustainable rice production,” Int. J. Biol. Macromol., vol. 168, pp. 834–845, 2021.
- P. L. Kashyap, X. Xiang, and P. Heiden, “Chitosan nanoparticle-based delivery systems for sustainable agriculture,” Int. J. Biol. Macromol., vol. 77, pp. 36–51, 2015.
- S. M. Ahsan, M. Thomas, K. K. Reddy, S. G. Sooraparaju, A. Asthana, and I. Bhatnagar, “Chitosan as a biomaterial in drug delivery and tissue engineering,” Int. J. Biol. Macromol., vol. 110, pp. 97–109, 2018.
- C. Vauthier, C. Zandanel, and A. L. Ramon, “Chitosan-based nanoparticles for in vivo delivery of interfering agents including siRNA,” Curr. Opin. Colloid Interface Sci., vol. 18, no. 5, pp. 406–418, 2013.
- N. Lal, J. Dubey, P. Gaur, N. Verma, and A. Verma, “Chitosan-based in situ forming polyelectrolyte complexes: A potential sustained drug delivery polymeric carrier for high dose drugs,” Mater. Sci. Eng. C, vol. 79, pp. 491–498, 2017.
- M. Prabaharan, “Chitosan-based nanoparticles for tumor-targeted drug delivery,” Int. J. Biol. Macromol., vol. 72, pp. 1313–1322, 2015.
- X. Zhang, X. Yang, J. Ji, A. Liu, and G. Zhai, “Tumor targeting strategies for chitosan-based nanoparticles,” Colloids Surfaces B Biointerfaces, vol. 148, pp. 460–473, 2016.
- S. Wei, Y. C. Ching, and C. H. Chuah, “Synthesis of chitosan aerogels as promising carriers for drug delivery: A review,” Carbohydr. Polym., vol. 231, no. November 2019, p. 115744, 2020.
- A. M. Olaru, L. Marin, S. Morariu, G. Pricope, M. Pinteala, and L. Tartau-Mititelu, “Biocompatible chitosan-based hydrogels for potential application in local tumor therapy,” Carbohydr. Polym., vol. 179, no. August 2017, pp. 59–70, 2018.
- R. Sedghi, M. Gholami, A. Shaabani, M. Saber, and H. Niknejad, “Preparation of novel chitosan derivative nanofibers for prevention of breast cancer recurrence,” Eur. Polym. J., vol. 123, p. 109421, 2020.
- A. Ali and S. Ahmed, “A review on chitosan and its nanocomposites in drug delivery,” Int. J. Biol. Macromol., vol. 109, pp. 273–286, 2018.
- R. Shanmuganathan, T. N. J. I. Edison, F. LewisOscar, P. Kumar, S. Shanmugam, and A. Pugazhendhi, “Chitosan nanopolymers: An overview of drug delivery against cancer,” Int. J. Biol. Macromol., vol. 130, pp. 727–736, 2019.
- C. Galo, S. Monsalve-Rozas, and L. Vergara-González, “Microencapsulation of Erlotinib and Nanomagnetite Supported in Chitosan as Potential Oncologic Carrier,” Polymers (Basel)., vol. 13, no. 1244, p. 22, 2021.
- Z. Shariatinia, “Pharmaceutical applications of chitosan,” Adv. Colloid Interface Sci., vol. 263, pp. 131–194, 2019.
- N. Islam, I. Damour, and M. O. Taha, “Degradability of chitosan micro/nanoparticles for pulmonary drug delivery,” Heliyon, vol. 5, no. 5, p. e01684, 2019.
- M. A. Mohammed, J. T. M. Syeda, K. M. Wasan, and E. K. Wasan, “An overview of chitosan nanoparticles and its application in non-parenteral drug delivery,” Pharmaceutics, vol. 9, no. 4, 2017.
- Y. Xiao and D. Yu, “Tumor microenvironment as a therapeutic target in cancer,” Pharmacol. Ther., vol. 221, p. 107753, 2021.
- J. Shim, J. Kang, and S. Il Yun, “Chitosan–dipeptide hydrogels as potential anticancer drug delivery systems,” Int. J. Biol. Macromol., vol. 187, no. July, pp. 399–408, 2021.
- S. Inphonlek, P. Sunintaboon, M. Léonard, and A. Durand, “Chitosan/carboxy methylcellulose-stabilized poly(lactide-co-glycolide) particles as bio-based drug delivery carriers,” Carbohydr. Polym., vol. 242, no. January, p. 116417, 2020.
- M. A. Khapre, S. Pandey, and R. M. Jugade, “Glutaraldehyde-cross-linked chitosan–alginate composite for organic dyes removal from aqueous solutions,” Int. J. Biol. Macromol., vol. 190, no. July, pp. 862–875, 2021.
- Y. Ma, K. J. Thurecht, and A. G. A. Coombes, “Development of enteric-coated, biphasic chitosan/HPMC microcapsules for colon-targeted delivery of anticancer drug-loaded nanoparticles,” Int. J. Pharm., vol. 607, no. June, p. 121026, 2021.
- F. Khatami, M. M. Martin, N. M. Danesh, A. R. Bahrami, K. Abnous, and S. M. Taghdisi, “Targeted delivery system using silica nanoparticles coated with chitosan and AS1411 for combination therapy of doxorubicin and antimiR-21,” Carbohydr. Polym., vol. 266, no. April, p. 118111, 2021.
- X. Chen et al., “Magnetic and self-healing chitosan-alginate hydrogel encapsulated gelatin microspheres via covalent cross-linking for drug delivery,” Mater. Sci. Eng. C, vol. 101, no. April, pp. 619–629, 2019.
- Z. Khorsandi, M. Borjian-Boroujeni, R. Yekani, and R. S. Varma, Carbon nanomaterials with chitosan: A winning combination for drug delivery systems. Elsevier B.V., 2021.
- S. A. Agnihotri, N. N. Mallikarjuna, and T. M. Aminabhavi, “Recent advances on chitosan-based micro-and nanoparticles in drug delivery,” J. Control. Release, vol. 100, no. 1, pp. 5–28, 2004.
- G. Cárdenas-Triviño, M. Burgos, and C. Von Plessing, “Microencapsulation of oxolinic acid with chitosan beads,” J. Chil. Chem. Soc., vol. 63, no. 4, pp. 4229–4238, 2018.
- S. Salalah and I. W. Lenggoro, “Nanoparticles carrying biological molecules: Recent advances and applications,” KONA Powder Part. J., vol. 2018, no. 35, pp. 89–111, 2018.
- S. Yu, X. Xu, J. Feng, M. Liu, and K. Hu, “Chitosan and chitosan coating nanoparticles for the treatment of brain disease,” Int. J. Pharm., vol. 560, no. February, pp. 282–293, 2019.
- E. Zhang, R. Xing, S. Liu, Y. Qin, K. Li, and P. Li, “Advances in chitosan-based nanoparticles for oncotherapy,” Carbohydr. Polym., vol. 222, no. June, p. 115004, 2019.
- G. P. Barbosa, H. S. Debone, P. Severino, E. B. Souto, and C. F. Da Silva, “Design and characterization of chitosan/zeolite composite films - Effect of zeolite type and zeolite dose on the film properties,” Mater. Sci. Eng. C, vol. 60, pp. 246–254, 2016.
- A. Khani Oushani, M. Soltani, N. Sheikhzadeh, M. Shamsaie Mehrgan, and H. Rajabi Islami, “Effects of dietary chitosan and nano-chitosan loaded clinoptilolite on growth and immune responses of rainbow trout (Oncorhynchus mykiss),” Fish Shellfish Immunol., vol. 98, no. July 2019, pp. 210–217, 2020.
- M. V. Dinu, A. I. Cocarta, and E. S. Dragan, “Synthesis, characterization and drug release properties of 3D chitosan/clinoptilolite biocomposite cryogels,” Carbohydr. Polym., vol. 153, pp. 203–211, 2016.
- X. Liu, X. Li, R. Zhang, L. Wang, and Q. Feng, “A novel dual microsphere-based on water-soluble thiolated chitosan/mesoporous calcium carbonate for controlled dual drug delivery,” Mater. Lett., vol. 285, p. 129142, 2021.
- L. Guo et al., “Combinatorial photothermal and Immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles,” ACS Nano, vol. 8, no. 6, pp. 5670–5681, 2014.
- H. Horo, S. Das, B. Mandal, and L. M. Kundu, “Development of a photoresponsive chitosan conjugated prodrug nano-carrier for controlled delivery of antitumor drug 5-fluorouracil,” Int. J. Biol. Macromol., vol. 121, pp. 1070–1076, 2019.
- A. Almeida et al., “Novel amphiphilic chitosan micelles as carriers for hydrophobic anticancer drugs,” Mater. Sci. Eng. C, vol. 112, no. November 2019, p. 110920, 2020.
- H. Tan, F. Qin, D. Chen, S. Han, W. Lu, and X. Yao, “Study of glycol chitosan-carboxymethyl β-cyclodextrins as anticancer drugs carrier,” Carbohydr. Polym., vol. 93, no. 2, pp. 679–685, 2013.
- F. Luo et al., “pH-responsive stearic acid-O-carboxymethyl chitosan assemblies as carriers delivering small molecular drug for chemotherapy,” Mater. Sci. Eng. C, vol. 105, no. August, p. 110107, 2019.
- R. Rajesh, M. R. Rekha, and C. P. Sharma, “Evaluation of lauryl chitosan graft polyethyleneimine as a potential carrier of genes and anticancer drugs,” Process Biochem., vol. 47, no. 7, pp. 1079–1088, 2012.
- H. Hosseinzadeh, F. Atyabi, R. Dinarvand, and S. N. Ostad, “Chitosan-Pluronic nanoparticles as oral delivery of anticancer gemcitabine: Preparation and in vitro study,” Int. J. Nanomedicine, vol. 7, pp. 1851–1863, 2012.
- R. Li et al., “Injectable halloysite-g-chitosan hydrogels as drug carriers to inhibit breast cancer recurrence,” Compos. Part B Eng., vol. 221, no. May, p. 109031, 2021.
- T. S. Anirudhan, C. Sekhar V., and S. S. Nair, “Polyelectrolyte complexes of carboxymethyl chitosan/alginate-based drug carrier for targeted and controlled release of a dual drug,” J. Drug Deliv. Sci. Technol., vol. 51, no. December 2018, pp. 569–582, 2019.
- R. P. Dhavale et al., “Chitosan coated magnetic nanoparticles as carriers of anticancer drug Telmisartan: pH-responsive controlled drug release and cytotoxicity studies,” J. Phys. Chem. Solids, vol. 148, no. July 2020, p. 109749, 2021.
- M. Parsian, G. Unsoy, P. Mutlu, S. Yalcin, A. Tezcaner, and U. Gunduz, “Loading of Gemcitabine on chitosan magnetic nanoparticles increase the anti-cancer efficacy of the drug,” Eur. J. Pharmacol., vol. 784, no. May, pp. 121–128, 2016.
- Q. Yuan, J. Shah, S. Hein, and R. D. K. Misra, “Controlled and extended drug release behavior of chitosan-based nanoparticle carrier,” Acta Biomater., vol. 6, no. 3, pp. 1140–1148, 2010.
- S. Kamaraj, U. M. Palanisamy, M. S. B. Kadhar Mohamed, A. Gangasalam, G. A. Maria, and R. Kandasamy, “Curcumin drug delivery by vanillin-chitosan coated with calcium ferrite hybrid nanoparticles as a carrier,” Eur. J. Pharm. Sci., vol. 116, no. April 2017, pp. 48–60, 2018.
- J. L. Arias, L. H. Reddy, and P. Couvreur, “Fe 3O 4/chitosan nanocomposite for magnetic drug targeting to cancer,” J. Mater. Chem., vol. 22, no. 15, pp. 7622–7632, 2012.
- G. Unsoy, R. Khodadust, S. Yalcin, P. Mutlu, and U. Gunduz, “Synthesis of Doxorubicin loaded magnetic chitosan nanoparticles for pH-responsive targeted drug delivery,” Eur. J. Pharm. Sci., vol. 62, pp. 243–250, 2014.
- N. Singh, G. J. S. Jenkins, R. Asadi, and S. H. Doak, “Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION),” Nano Rev., vol. 1, no. 1, p. 5358, 2010.
- L. Xie, W. Jin, H. Chen, and Q. Zhang, “Superparamagnetic iron oxide nanoparticles for cancer diagnosis and therapy,” J. Biomed. Nanotechnol., vol. 15, no. 2, pp. 215–235, 2019.
- R. Rajalakshmi, S. Sivaselvam, and N. Ponpandian, “Chitosan grafted Fe-doped WO3 decorated with gold nanoparticles for stimuli-responsive drug delivery systems,” Mater. Lett., vol. 304, no. March, p. 130664, 2021.
- A. Almeida, V. Linares, G. Mora-Castaño, M. Casas, I. Caraballo, and B. Sarmento, “3D printed systems for colon-specific delivery of camptothecin-loaded chitosan micelles,” Eur. J. Pharm. Biopharm., vol. 167, no. June, pp. 48–56, 2021.
- G. Lohiya and D. S. Katti, “Carboxylated chitosan mediated improved efficacy of mesoporous silica nanoparticle-based targeted drug delivery system for breast cancer therapy,” Carbohydr. Polym., p. 118822, 2021.
- S. Maya, B. Sarmento, V. K. Lakshmanan, D. Menon, V. Seabra, and R. Jayakumar, “Chitosan cross-linked docetaxel loaded EGF receptor-targeted nanoparticles for lung cancer cells,” Int. J. Biol. Macromol., vol. 69, pp. 532–541, 2014.
- L. Khoshtabiat, A. Meshkini, and M. M. Martin, “Fenton-magnetic based therapy by dual-chemo drug-loaded magnetic hydroxyapatite against colon cancer,” Mater. Sci. Eng. C, vol. 127, no. February, p. 112238, 2021.
- L. Tao, J. Jiang, Y. Gao, C. Wu, and Y. Liu, “Biodegradable Alginate-Chitosan Hollow Nanospheres for Codelivery of Doxorubicin and Paclitaxel for the Effect of Human Lung Cancer A549 Cells,” Biomed Res. Int., vol. 2018, 2018.
- A. B. Nazlı and Y. S. Açıkel, “Loading of cancer drug resveratrol to pH-Sensitive, smart, alginate-chitosan hydrogels and investigation of controlled release kinetics,” J. Drug Deliv. Sci. Technol., vol. 53, no. April, p. 101199, 2019.
- D. Qu et al., “Anisamide-functionalized pH-responsive amphiphilic chitosan-based paclitaxel micelles for sigma-1 receptor-targeted prostate cancer treatment,” Carbohydr. Polym., vol. 229, p. 115498, 2020.
- M. Fathi, J. Barar, H. Erfan-Niya, and Y. Omidi, “Methotrexate-conjugated chitosan-grafted pH- and thermo-responsive magnetic nanoparticles for targeted therapy of ovarian cancer,” Int. J. Biol. Macromol., vol. 154, pp. 1175–1184, 2020.
- L. Zhao et al., “Chitosan/Sulfobutylether-β-Cyclodextrin Nanoparticles for Ibrutinib Delivery: A Potential Nanoformulation of Novel Kinase Inhibitor,” J. Pharm. Sci., vol. 109, no. 2, pp. 1136–1144, 2020.
- S. Bhattacharya, “Fabrication and characterization of chitosan-based polymeric nanoparticles of Imatinib for colorectal cancer targeting application,” Int. J. Biol. Macromol., vol. 151, pp. 104–115, 2020.
- “Home - ClinicalTrials.gov.” [Online]. Available: https://www.clinicaltrials.gov/. [Accessed: 14-Nov-2021].