- Cosmetics,
- Heavy metals,
- Mercury,
- Lead,
- Cadmium
- Chromium ...More
Copyright (c) 2022 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
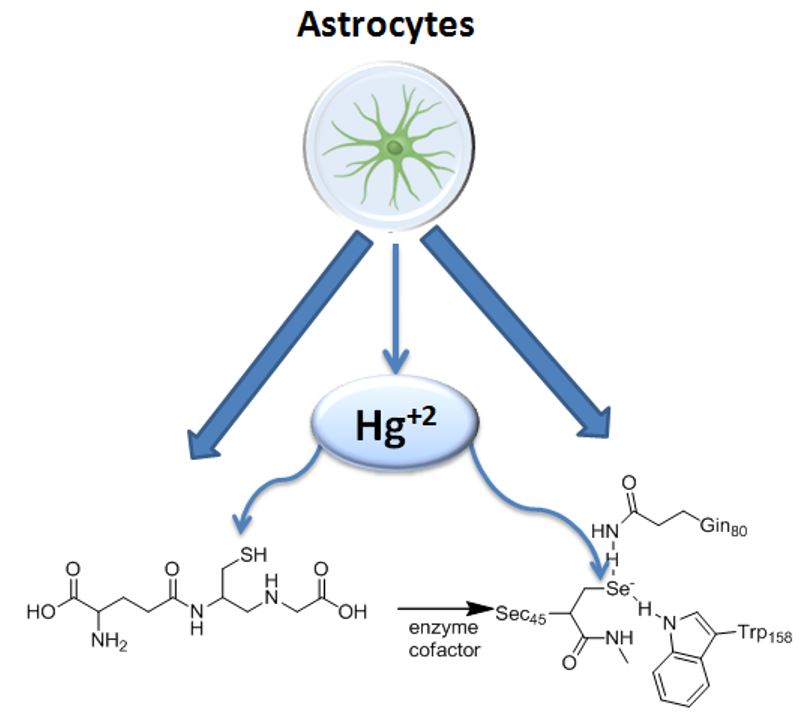
Cosmetics have been used by humans since the start of human civilization. Initially, it was typically consisting of natural products but to get prompt results, heavy metals were frequently added to cosmetics to accelerate the affects. Heavy metals such as mercury (Hg), lead (Pb), cadmium (Cd) and chromium (Cr) are detected in various cosmetic products; most frequently color cosmetics, herbal cosmetics, hair cosmetics, face-body care products and beauty cosmetic products. These metals are included in toxic metals. The application of cosmetics to different body parts leads to the absorption of metals through the stratum corneum into the blood, accumulate or replace essential elements of different biomolecules which triggers the unfavorable effects. Reported data show that in some common cosmetic products toxic metals may be present greater than permissible limit. The United States Food and Drug Administration (FDA) and World Health Organization (WHO) have established the highest permissible limit of exposure for heavy metals in different cosmetic products. However, commonly in developing world no care is taken for permissible limit set by FDA and WHO and to obey cosmetic production legislations which resulted in fatal health consequences. Thus, in this review article we are focusing on the permissible limits, hazardous effects of the toxic metals and mechanisms associated with the hazardous effects related to heavy metals found in cosmetics. Owing to the growing usage of cosmetics it is necessary to explore the possible sources and routs of metals toxicity to fix the hazardous effects related to heavy metals found in cosmetics.
References
- H. Malvandi, and F. Sancholi, “Assessments of some metals contamination in lipsticks and their associated health risks to lipstick consumers in Iran,” Environmental monitoring and assessment, vol. 190, no. 11, pp. 680, 2018.
- P. Worsfold, A. Townshend, C. F. Poole, and M. Miró, Encyclopedia of analytical science: Elsevier, 2019.
- J. Okereke, A. Udebuani, E. Ezeji, K. Obasi, and M. Nnoli, “Possible health implications associated with cosmetics: a review,” Sci J Public Health, vol. 3, no. 5-1, pp. 58-63, 2015.
- C. M. Iwegbue, F. I. Bassey, G. Obi, G. O. Tesi, and B. S. Martincigh, “Concentrations and exposure risks of some metals in facial cosmetics in Nigeria,” Toxicology Reports, vol. 3, pp. 464-472, 2016.
- S. Borowska, and M. M. Brzóska, “Metals in cosmetics: implications for human health,” Journal of applied toxicology, vol. 35, no. 6, pp. 551-572, 2015.
- S. Y. Ng, F. Dewi, J. Wang, L. P. Sim, R. Y. C. Shin, and T. K. Lee, “Development of a cosmetic cream certified reference material: Certification of lead, mercury and arsenic mass fractions in cosmetic cream,” International Journal of Mass Spectrometry, vol. 389, pp. 59-65, 2015/10/15/, 2015.
- S. Y. Ng, F. Dewi, J. Wang, L. P. Sim, R. Y. Shin, and T. K. Lee, “Development of a cosmetic cream certified reference material: Certification of lead, mercury and arsenic mass fractions in cosmetic cream,” International Journal of Mass Spectrometry, vol. 389, pp. 59-65, 2015.
- A. Massadeh, M. El-Khateeb, and S. Ibrahim, “Evaluation of Cd, Cr, Cu, Ni, and Pb in selected cosmetic products from Jordanian, Sudanese, and Syrian markets,” Public health, vol. 149, pp. 130-137, 2017.
- B. Kaličanin, and D. Velimirović, “A study of the possible harmful effects of cosmetic beauty products on human health,” Biological trace element research, vol. 170, no. 2, pp. 476-484, 2016.
- R. Kroes, A. Renwick, V. Feron, C. Galli, M. Gibney, H. Greim, R. Guy, J. Lhuguenot, and J. Van de Sandt, “Application of the threshold of toxicological concern (TTC) to the safety evaluation of cosmetic ingredients,” Food and Chemical Toxicology, vol. 45, no. 12, pp. 2533-2562, 2007.
- G. Forte, F. Petrucci, and B. Bocca, “Metal allergens of growing significance: epidemiology, immunotoxicology, strategies for testing and prevention,” Inflammation & Allergy-Drug Targets (Formerly Current Drug Targets-Inflammation & Allergy)(Discontinued), vol. 7, no. 3, pp. 145-162, 2008.
- G. J. Nohynek, E. Antignac, T. Re, and H. Toutain, “Safety assessment of personal care products/cosmetics and their ingredients,” Toxicology and applied pharmacology, vol. 243, no. 2, pp. 239-259, 2010.
- A. Zakaria, and Y. B. Ho, “Heavy metals contamination in lipsticks and their associated health risks to lipstick consumers,” Regulatory toxicology and pharmacology, vol. 73, no. 1, pp. 191-195, 2015.
- P. Onojah, and J. Emurotu, “Heavy metals in selected skin lighting creams and medicated soaps,” International Journal of Innovation in Science and Mathematics, vol. 5, no. 3, 2017.
- A. L. Luz, X. Wu, and E. J. Tokar, "Toxicology of Inorganic Carcinogens," Advances in Molecular Toxicology, pp. 1-46: Elsevier, 2018.
- Q. Zhang, L. Zhang, X. Xiao, Z. Su, P. Zou, H. Hu, Y. Huang, and Q.-Y. He, “Heavy metals chromium and neodymium reduced phosphorylation level of heat shock protein 27 in human keratinocytes,” Toxicology In Vitro, vol. 24, no. 4, pp. 1098-1104, 2010.
- R. Ridzwan, and B. H. Zainudin, “Contact Allergic Dermatitis to Cosmetics and Topical Anti-ageing Products,” Malaysian J Dermatol, vol. 39, pp. 10-21, 2017.
- S. Agrawal, and P. Sharma, “Current status of mercury level in skin whitening creams,” Current Medicine Research and Practice, vol. 7, no. 2, pp. 47-50, 2017.
- D. S. Lim, T. H. Roh, M. K. Kim, Y. C. Kwon, S. M. Choi, S. J. Kwack, K. B. Kim, S. Yoon, H. S. Kim, and B.-M. Lee, “Non-cancer, cancer, and dermal sensitization risk assessment of heavy metals in cosmetics,” Journal of Toxicology and Environmental Health, Part A, vol. 81, no. 11, pp. 432-452, 2018.
- A. Sani, M. B. Gaya, and F. A. Abubakar, “Determination of some heavy metals in selected cosmetic products sold in kano metropolis, Nigeria,” Toxicology reports, vol. 3, pp. 866-869, 2016.
- M. Jaishankar, T. Tseten, N. Anbalagan, B. B. Mathew, and K. N. Beeregowda, “Toxicity, mechanism and health effects of some heavy metals,” Interdisciplinary toxicology, vol. 7, no. 2, pp. 60-72, 2014.
- D. E. Engler, “Mercury "bleaching" creams,” Journal of the American Academy of Dermatology, vol. 52, no. 6, pp. 1113-1114, 2005.
- UNEP, “Text and Annexes, Minamata Convention on Mercury, Nairobi, United Nations Environment Programme,” 2019. http://www.mercuryconvention.org/Convention/Text/tabid/3426/language/en-US/Default.aspx.
- E. Uram, B. Bischofer, and S. Hagemann, “Market analysis of some mercury-containing products and their mercury-free alternatives in selected regions,” Gesellschaft für Anlagenund Reaktorsicherheit (GRS) mbH, March (GRS-253), 2010. http://ipen.org/sites/default/files/documents/market_analysis_mercurycontaining_products_alternatives-en.pdf.
- H. Shroff, P. C. Diedrichs, and N. Craddock, “Skin Color, Cultural Capital, and Beauty Products: An Investigation of the Use of Skin Fairness Products in Mumbai, India,” Frontiers in Public Health, vol. 5, no. 365, 2018-January-23, 2018.
- O. Z. Moraa, “Levels of selected heavy metal in aloe vera branded skin cosmetics,” Master thesis, 2014.
- J. Chen, Y. Ye, M. Ran, Q. Li, Z. Ruan, and N. Jin, “Inhibition of Tyrosinase by Mercury Chloride: Spectroscopic and Docking Studies,” Frontiers in pharmacology, vol. 11, pp. 81-81, 2020.
- S. S. Agrawal, and M. Mazhar, “Adulteration of mercury in skin whitening creams – A nephrotoxic agent,” Current Medicine Research and Practice, vol. 5, no. 4, pp. 172-175, 2015/07/01/, 2015.
- S. S. Agrawal, and P. Sharma, “Current status of mercury level in skin whitening creams,” Current Medicine Research and Practice, vol. 7, no. 2, pp. 47-50, 2017/03/01/, 2017.
- Y. O.-Y. Soo, K.-M. Chow, C. W.-K. Lam, F. M.-M. Lai, C.-C. Szeto, M. H.-M. Chan, and P. K.-T. Li, “A whitened face woman with nephrotic syndrome,” American journal of kidney diseases, vol. 41, no. 1, pp. 250-253, 2003.
- D. Gonzalez-Ramirez, M. Zuniga-Charles, A. Narro-Juarez, Y. Molina-Recio, K. M. Hurlbut, R. C. Dart, and H. V. Aposhian, “DMPS (2, 3-dimercaptopropane-1-sulfonate, dimaval) decreases the body burden of mercury in humans exposed to mercurous chloride,” Journal of Pharmacology and Experimental Therapeutics, vol. 287, no. 1, pp. 8-12, 1998.
- H. V. Aposhian, “Mobilization of mercury and arsenic in humans by sodium 2, 3-dimercapto-1-propane sulfonate (DMPS),” Environmental Health Perspectives, vol. 106, no. suppl 4, pp. 1017-1025, 1998.
- M. Blanusa, V. M. Varnai, M. Piasek, and K. Kostial, “Chelators as antidotes of metal toxicity: therapeutic and experimental aspects,” Current medicinal chemistry, vol. 12, no. 23, pp. 2771-2794, 2005.
- A. R. Zota, and B. Shamasunder, “The environmental injustice of beauty: framing chemical exposures from beauty products as a health disparities concern,” American journal of obstetrics and gynecology, vol. 217, no. 4, pp. 418. e1-418. e6, 2017.
- M. R. Ori, and J. B. Larsen, “Mercury Poisoning in a Toddler from Home Contamination due to Skin-Lightening Cream,” The Journal of pediatrics, vol. 196, pp. 314-317. e1, 2018.
- L. Zhang, F. Liu, Y. Peng, L. Sun, and C. Chen, “Nephrotic syndrome of minimal change disease following exposure to mercury-containing skin-lightening cream,” Annals of Saudi medicine, vol. 34, no. 3, pp. 257-261, May-Jun, 2014.
- S. Agrawal, and M. Mazhar, “Adulteration of mercury in skin whitening creams–A nephrotoxic agent,” Current Medicine Research and Practice, vol. 5, no. 4, pp. 172-175, 2015.
- H. X. Niu, S. H. Li, H. Y. Li, Y. H. Chen, W. W. Liu, P. L. Li, and H. B. Long, “Clinicopathological features, diagnosis, and treatment of IgA nephropathy with minimal change disease related to exposure to mercury-containing cosmetics: a case report ” Clin Nephrol, vol. 87 (2017), no. 4, pp. 196-201, Apr, 2017.
- T. Y. K. Chan, A. P. L. Chan, and H. L. Tang, “Nephrotic syndrome caused by exposures to skin-lightening cosmetic products containing inorganic mercury,” Clin Toxicol (Phila), vol. 58, no. 1, pp. 9-15, Jan, 2020.
- M. N. Rana, J. Tangpong, and M. M. Rahman, “Toxicodynamics of lead, cadmium, mercury and arsenic-induced kidney toxicity and treatment strategy: a mini review,” Toxicology reports, vol. 5, pp. 704-713, 2018.
- T. Y. Chan, “Inorganic mercury poisoning associated with skin-lightening cosmetic products,” Clinical toxicology, vol. 49, no. 10, pp. 886-891, 2011.
- G. Bjørklund, J. Aaseth, O. P. Ajsuvakova, A. A. Nikonorov, A. V. Skalny, M. G. Skalnaya, and A. A. Tinkov, “Molecular interaction between mercury and selenium in neurotoxicity,” Coordination Chemistry Reviews, vol. 332, pp. 30-37, 2017.
- S. K. Nigam, K. T. Bush, G. Martovetsky, S.-Y. Ahn, H. C. Liu, E. Richard, V. Bhatnagar, and W. Wu, “The organic anion transporter (OAT) family: a systems biology perspective,” Physiological reviews, vol. 95, no. 1, pp. 83-123, 2015.
- R. Zalups, and C. Bridges, “Mechanisms Involved in the Renal Handling and Toxicity of Mercury,” 2018.
- I. J. Kade, “Mercury toxicity on sodium pump and organoseleniums intervention: a paradox,” BioMed Research International, vol. 2012, 2012.
- A. D. Monnot, W. V. Christian, M. M. Abramson, and M. H. Follansbee, “An exposure and health risk assessment of lead (Pb) in lipstick,” Food and Chemical Toxicology, vol. 80, pp. 253-260, 2015.
- G. Flora, D. Gupta, and A. Tiwari, “Toxicity of lead: A review with recent updates,” Interdisciplinary toxicology, vol. 5, no. 2, pp. 47-58, 2012.
- K. Nemsadze, T. Sanikidze, L. Ratiani, L. Gabunia, and T. Sharashenidze, “Mechanisms of lead-induced poisoning,” Georgian Med News, no. 172-173, pp. 92-6, Jul-Aug, 2009.
- T. Sanders, Y. Liu, V. Buchner, and P. B. Tchounwou, “Neurotoxic effects and biomarkers of lead exposure: a review,” Reviews on environmental health, vol. 24, no. 1, pp. 15-45, Jan-Mar, 2009.
- M. A. Al-Qutob, H. M. Alatrash, and S. Abol-Ola, “Determination of different heavy metals concentrations in cosmetics purchased from the Palestinian markets by ICP/MS,” 2013.
- E. Stojek, and A. Skoczyńska, “[Lead effect on vascular endothelium],” Med Pr, vol. 54, no. 1, pp. 87-93, 2003.
- V. Matović, A. Buha, D. Ðukić-Ćosić, and Z. Bulat, “Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys,” Food and Chemical Toxicology, vol. 78, pp. 130-140, 2015/04/01/, 2015.
- E. J. Bechara, “Oxidative stress in acute intermittent porphyria and lead poisoning may be triggered by 5-aminolevulinic acid,” Braz J Med Biol Res, vol. 29, no. 7, pp. 841-51, Jul, 1996.
- A. Kasperczyk, G. Machnik, M. Dobrakowski, D. Sypniewski, E. Birkner, and S. Kasperczyk, “Gene expression and activity of antioxidant enzymes in the blood cells of workers who were occupationally exposed to lead,” Toxicology, vol. 301, no. 1-3, pp. 79-84, Nov 15, 2012.
- A. Wilk, E. Kalisińska, D. I. Kosik-Bogacka, M. Romanowski, J. Różański, K. Ciechanowski, M. Słojewski, and N. Łanocha-Arendarczyk, “Cadmium, lead and mercury concentrations in pathologically altered human kidneys,” Environmental geochemistry and health, vol. 39, no. 4, pp. 889-899, 2017.
- S. J. Cobbina, Y. Chen, Z. Zhou, X. Wu, T. Zhao, Z. Zhang, W. Feng, W. Wang, Q. Li, and X. Wu, “Toxicity assessment due to sub-chronic exposure to individual and mixtures of four toxic heavy metals,” Journal of hazardous materials, vol. 294, pp. 109-120, 2015.
- M. A. Assi, M. N. M. Hezmee, A. W. Haron, M. Y. M. Sabri, and M. A. Rajion, “The detrimental effects of lead on human and animal health,” Veterinary world, vol. 9, no. 6, pp. 660, 2016.
- X. Wu, S. J. Cobbina, G. Mao, H. Xu, Z. Zhang, and L. Yang, “A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment,” Environmental Science and Pollution Research, vol. 23, no. 9, pp. 8244-8259, 2016.
- A. Oskarsson, L. Olson, M. R. Palmer, B. Lind, H. Björklund, and B. Hoffer, “Increased lead concentration in brain and potentiation of lead-induced neuronal depression in rats after combined treatment with lead and disulfiram,” Environmental research, vol. 41, no. 2, pp. 623-632, 1986.
- R. Bull, S. Lutkenhoff, G. McCarty, and R. Miller, “Delays in the postnatal increase of cerebral cytochrome concentrations in lead-exposed rats,” Neuropharmacology, vol. 18, no. 1, pp. 83-92, 1979.
- L. C. Schenkel, K. D. Kernohan, A. McBride, D. Reina, A. Hodge, P. J. Ainsworth, D. I. Rodenhiser, G. Pare, N. G. Bérubé, C. Skinner, K. M. Boycott, C. Schwartz, and B. Sadikovic, “Identification of epigenetic signature associated with alpha thalassemia/mental retardation X-linked syndrome,” Epigenetics & Chromatin, vol. 10, no. 1, pp. 10, 2017/03/10, 2017.
- S.-Y. Kwon, O.-N. Bae, J.-Y. Noh, K. Kim, S. Kang, Y.-J. Shin, K.-M. Lim, and J.-H. Chung, “Erythrophagocytosis of lead-exposed erythrocytes by renal tubular cells: possible role in lead-induced nephrotoxicity,” Environmental health perspectives, vol. 123, no. 2, pp. 120-127, 2014.
- P. McCauley, R. Bull, and S. Lutkenhoff, “Association of alterations in energy metabolism with lead-induced delays in rat cerebral cortical development,” Neuropharmacology, vol. 18, no. 1, pp. 93-101, 1979.
- K. A. Allen, and S. Gephart, “Is prenatal lead exposure a concern in infancy? What is the evidence?,” Advances in neonatal care, vol. 15, no. 6, pp. 416-420, 2015.
- A. Lafuente, “The hypothalamic–pituitary–gonadal axis is target of cadmium toxicity. An update of recent studies and potential therapeutic approaches,” Food and Chemical Toxicology, vol. 59, pp. 395-404, 2013/09/01/, 2013.
- P. C. Chen, I. J. Pan, and J. D. Wang, “Parental exposure to lead and small for gestational age births,” American journal of industrial medicine, vol. 49, no. 6, pp. 417-422, 2006.
- L. Cheng, B. Zhang, W. Huo, Z. Cao, W. Liu, J. Liao, W. Xia, S. Xu, and Y. Li, “Fetal exposure to lead during pregnancy and the risk of preterm and early-term deliveries,” International journal of hygiene and environmental health, vol. 220, no. 6, pp. 984-989, 2017.
- S. Elmore, “Apoptosis: a review of programmed cell death,” Toxicologic pathology, vol. 35, no. 4, pp. 495-516, 2007.
- A. L. Wani, A. Ara, and J. A. Usmani, “Lead toxicity: a review,” Interdisciplinary toxicology, vol. 8, no. 2, pp. 55-64, 2015.
- M. D. Nye, K. E. King, T. H. Darrah, R. Maguire, D. D. Jima, Z. Huang, M. A. Mendez, R. C. Fry, R. L. Jirtle, and S. K. Murphy, “Maternal blood lead concentrations, DNA methylation of MEG3 DMR regulating the DLK1/MEG3 imprinted domain and early growth in a multiethnic cohort,” Environmental epigenetics, vol. 2, no. 1, pp. dvv009, 2016.
- G. F. Nordberg, “Historical perspectives on cadmium toxicology,” Toxicol Appl Pharmacol, vol. 238, no. 3, pp. 192-200, Aug 1, 2009.
- C. Fasanya-Odewumi, L. M. Latinwo, C. O. Ikediobi, L. Gilliard, G. Sponholtz, J. Nwoga, F. Stino, N. Hamilton, and G. W. Erdos, “The genotoxicity and cytotoxicity of dermally-administered cadmium: effects of dermal cadmium administration,” Int J Mol Med, vol. 1, no. 6, pp. 1001-6, Jun, 1998.
- O. Orisakwe, "Other heavy metals: antimony, cadmium, chromium and mercury," Toxicity of building materials, pp. 297-333: Elsevier, 2012.
- I. Yunusa, M. Ibrahim, H. Yakasai, I. Ahmad, C. Odo, Z. Gidado, Z. Rabiu, N. Kabir, and L. Ezeanyika, “Heavy metals in female adolescents,” Age (years), vol. 1, pp. 0.31.
- A. Åkesson, T. Lundh, M. Vahter, P. Bjellerup, J. Lidfeldt, C. Nerbrand, G. Samsioe, U. Strömberg, and S. Skerfving, “Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure,” Environmental health perspectives, vol. 113, no. 11, pp. 1627-1631, 2005.
- N. Johri, G. Jacquillet, and R. Unwin, “Heavy metal poisoning: the effects of cadmium on the kidney,” Biometals, vol. 23, no. 5, pp. 783-792, 2010.
- M. Trzcinka-Ochocka, M. Jakubowski, G. Razniewska, T. Halatek, and A. Gazewski, “The effects of environmental cadmium exposure on kidney function: the possible influence of age,” Environmental research, vol. 95, no. 2, pp. 143-150, 2004.
- A. Åkesson, P. Bjellerup, T. Lundh, J. Lidfeldt, C. Nerbrand, G. Samsioe, S. Skerfving, and M. Vahter, “Cadmium-induced effects on bone in a population-based study of women,” Environmental health perspectives, vol. 114, no. 6, pp. 830-834, 2006.
- T. Jin, G. Nordberg, T. Ye, M. Bo, H. Wang, G. Zhu, Q. Kong, and A. Bernard, “Osteoporosis and renal dysfunction in a general population exposed to cadmium in China,” Environmental research, vol. 96, no. 3, pp. 353-359, 2004.
- M. H. Bhattacharyya, “Cadmium osteotoxicity in experimental animals: mechanisms and relationship to human exposures,” Toxicology and applied pharmacology, vol. 238, no. 3, pp. 258-265, 2009.
- H. Yokota, and H. Tonami, “Experimental studies on the bone metabolism of male rats chronically exposed to cadmium intoxication using dual-energy X-ray absorptiometry,” Toxicology and industrial health, vol. 24, no. 3, pp. 161-170, 2008.
- M. Trzcinka-Ochocka, M. Jakubowski, W. Szymczak, B. Janasik, and R. Brodzka, “The effects of low environmental cadmium exposure on bone density,” Environmental research, vol. 110, no. 3, pp. 286-293, 2010.
- “Scientific Opinion on Dietary Reference Values for chromium",” European Food Safety Authority. 18 September 2014; Retrieved 06 March 2021.
- Z. Wang, and C. Yang, "Metal carcinogen exposure induces cancer stem cell-like property through epigenetic reprograming: a novel mechanism of metal carcinogenesis."
- Z. Wang, and C. Yang, “Metal carcinogen exposure induces cancer stem cell-like property through epigenetic reprograming: A novel mechanism of metal carcinogenesis,” Semin Cancer Biol, vol. 57, pp. 95-104, Aug, 2019.
- J. P. Thyssen, J. D. Johansen, and T. Menné, “Contact allergy epidemics and their controls,” Contact Dermatitis, vol. 56, no. 4, pp. 185-95, Apr, 2007.
- H. Sun, J. Brocato, and M. Costa, “Oral Chromium Exposure and Toxicity,” Current environmental health reports, vol. 2, no. 3, pp. 295-303, 2015.
- J. B. Vincent, Y. Neggers, and J. McClung, "Roles of Chromium (III), Vanadium, Iron, and Zinc in Sports Nutrition," Nutrition and Enhanced Sports Performance, pp. 653-664: Elsevier, 2019.
- M. Hwang, E. K. Yoon, J. Y. Kim, B. K. Son, S. J. Yang, M. O. Yun, S. S. Choi, D. D. Jang, and T. M. Yoo, “Safety assessment of chromium by exposure from cosmetic products,” Archives of pharmacal research, vol. 32, no. 2, pp. 235-241, 2009.
- Z. Grosser, L. Davidowski, and L. Thompson, “The determination of metals in cosmetics,” PerkinElmer Appl. Note, pp. 1–6, 2011.
- M. A. Gondal, Z. S. Seddigi, M. M. Nasr, and B. Gondal, “Spectroscopic detection of health hazardous contaminants in lipstick using Laser Induced Breakdown Spectroscopy,” J Hazard Mater, vol. 175, no. 1-3, pp. 726-32, Mar 15, 2010.
- E. K. Kang, S. Lee, J. H. Park, K. M. Joo, H. J. Jeong, and I. S. Chang, “Determination of hexavalent chromium in cosmetic products by ion chromatography and postcolumn derivatization,” Contact Dermatitis, vol. 54, no. 5, pp. 244-8, May, 2006.
- E. L. Sainio, R. Jolanki, E. Hakala, and L. Kanerva, “Metals and arsenic in eye shadows,” Contact Dermatitis, vol. 42, no. 1, pp. 5-10, Jan, 2000.
- L. Sneyers, L. Verheyen, P. Vermaercke, and M. Bruggeman, “Trace element determination in beauty products by k0-instrumental neutron activation analysis,” Journal of Radioanalytical and Nuclear Chemistry, vol. 281, no. 2, pp. 259-263, 01 Aug. 2009, 2009.
- M. Corazza, F. Baldo, A. Pagnoni, R. Miscioscia, and A. Virgili, “Measurement of nickel, cobalt and chromium in toy make-up by atomic absorption spectroscopy,” Acta Derm Venereol, vol. 89, no. 2, pp. 130-3, 2009.
- C. F. Allenby, and B. F. Goodwin, “Influence of detergent washing powders on minimal eliciting patch test concentrations of nickel and chromium,” Contact Dermatitis, vol. 9, no. 6, pp. 491-9, Nov, 1983.
- C. F. Allenby, and D. A. Basketter, “Minimum eliciting patch test concentrations of cobalt,” Contact Dermatitis, vol. 20, no. 3, pp. 185-90, Mar, 1989.
- M. F. Alam, M. Akhter, B. Mazumder, A. Ferdous, M. D. Hossain, N. C. Dafader, F. T. Ahmed, S. K. Kundu, T. Taheri, and A. K. M. Atique Ullah, “Assessment of some heavy metals in selected cosmetics commonly used in Bangladesh and human health risk,” Journal of Analytical Science and Technology, vol. 10, no. 1, pp. 2, 2019/01/08, 2019.
- S. C. Chen, and C. M. Liao, “Health risk assessment on human exposed to environmental polycyclic aromatic hydrocarbons pollution sources,” Sci Total Environ, vol. 366, no. 1, pp. 112-23, Jul 31, 2006.