- Aluminium alloy,
- corrosion,
- plant extract,
- extraction method,
- adsorption isotherm
- mechanism ...More
Copyright (c) 2022 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
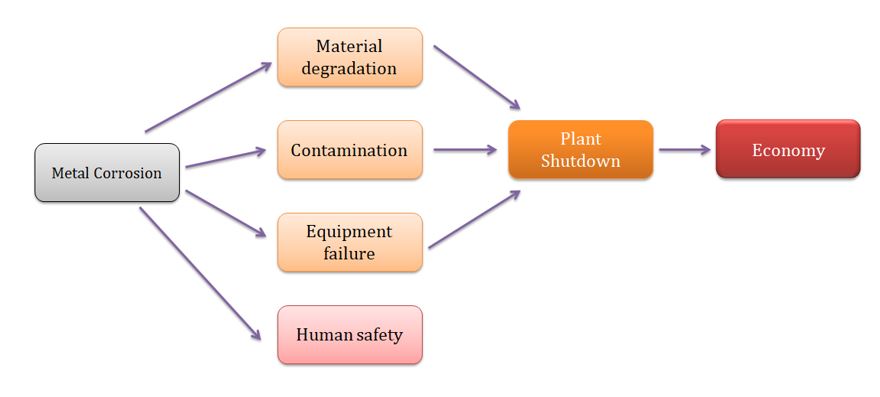
In spite of the fact that aluminium and its combinations are regularly used like lightweight materials in a great deal of enterprises, they experience the ill effects of low erosion resistance. Corrosion is a natural phenomena that leads to the deterioration of the metal properties through its electrochemical interaction with the corrosive environment. The costs related to corrosion can be either direct (due to the replacement and maintenance) or indirect. A few strategies and methods have been utilized to mitigate corrosion in aluminium and its compounds under various conditions. Concerns raised over climate and human wellbeing have constrained industries to search for a more appropriate option for the protection of aluminium and its alloys from corrosion. This review emphasizes on the plant extracts applied for aluminium combination consumption restraint in NaCl medium. It summarizes the different techniques used for extraction. Additionally, an understanding to the adsorption isotherms has been discussed in brief.
References
- Kadri Y, Srasra E, Bekri-Abbes I, Herrasti P (2021). Facile and eco-friendly synthesis of polyaniline/ZnO composites for corrosion protection of AA-2024 aluminium alloy. J Electroanal Chem, 893: 115335. https://doi.org/10.1016/j.jelechem.2021.115335
- Rosliza R, Wan Nik WB, Izman S, Prawoto Y (2010). Anti-corrosive properties of natural honey on Al–Mg–Si alloy in seawater. Curr Appl Phys, 10: 923–929. https://doi.org/10.1016/j.cap.2009.11.074
- Joseph OO, Joseph OO (2021). Corrosion Inhibition of Aluminium Alloy by Chemical Inhibitors: An Overview. IOP Conf Ser Mater Sci Eng, 1107: 012170. https://doi.org/10.1088/1757-899X/1107/1/012170
- Verdalet-Guardiola X, Bonino J-P, Duluard S, et al (2018). Influence of the alloy microstructure and surface state on the protective properties of trivalent chromium coatings grown on a 2024 aluminium alloy. Surf Coatings Technol, 344: 276–287. https://doi.org/10.1016/j.surfcoat.2018.03.046
- Li L, Doran KP, Swain GM (2013). Electrochemical Characterization of Trivalent Chromium Process (TCP) Coatings on Aluminum Alloys 6061 and 7075. J Electrochem Soc, 160: C396–C401. https://doi.org/10.1149/2.117308jes
- Verma C, Ebenso EE, Bahadur I, Quraishi MA (2018). An overview on plant extracts as environmental sustainable and green corrosion inhibitors for metals and alloys in aggressive corrosive media. J Mol Liq, 266: 577–590. https://doi.org/10.1016/j.molliq.2018.06.110
- Hu J, Xiong Q, Chen L, et al (2021). Corrosion inhibitor in CO2-O2-containing environment: Inhibition effect and mechanisms of Bis(2-ehylhexyl) phosphate for the corrosion of carbon steel. Corros Sci, 179: 109173. https://doi.org/10.1016/j.corsci.2020.109173
- Kamali Ardakani E, Kowsari E, Ehsani A (2020). Imidazolium-derived polymeric ionic liquid as a green inhibitor for corrosion inhibition of mild steel in 1.0 M HCl: Experimental and computational study. Colloids Surfaces A Physicochem Eng Asp, 586: 124195. https://doi.org/10.1016/j.colsurfa.2019.124195
- Boughoues Y, Benamira M, Messaadia L, Ribouh N (2020). Adsorption and corrosion inhibition performance of some environmental friendly organic inhibitors for mild steel in HCl solution via experimental and theoretical study. Colloids Surfaces A Physicochem Eng Asp, 593: 124610. https://doi.org/10.1016/j.colsurfa.2020.124610
- He J, Xu Q, Li G, et al (2021). Insight into the corrosion inhibition property of Artocarpus heterophyllus Lam leaves extract. J Ind Eng Chem, 102: 260–270. https://doi.org/10.1016/j.jiec.2021.07.007
- Suresh Kumar S, Kakooei S, Ismail MC, Haris M (2020). Synthesis and characterization of metal ion end capped nanocontainer loaded with duo green corrosion inhibitors. J Mater Res Technol, 9: 8350–8354. https://doi.org/10.1016/j.jmrt.2020.05.110
- Dehghani A, Bahlakeh G, Ramezanzadeh B, Ramezanzadeh M (2020). Potential role of a novel green eco-friendly inhibitor in corrosion inhibition of mild steel in HCl solution: Detailed macro/micro-scale experimental and computational explorations. Constr Build Mater, 245: 118464. https://doi.org/10.1016/j.conbuildmat.2020.118464
- Jeon H, Lim C, Lee JM, Kim S (2015). Chemical assay-guided natural product isolation via solid-supported chemodosimetric fluorescent probe. Chem Sci, 6: 2806–2811. https://doi.org/10.1039/C5SC00360A
- Kesavan D, Gopiraman M, Sulochana N (2012). Green Inhibitors for Corrosion of Metals : A Review Correspondence : Chem Sci Rev Lett
- Edraki M, Banimahd Keivani M (2020). Eco-friendly inhibitors for corrosion protection of metallic surfaces–a mini review. Asian J Green Chem, 4: 283–296
- Rana A, Arfaj MK, Saleh TA (2019). Advanced developments in shale inhibitors for oil production with low environmental footprints – A review. Fuel, 247: 237–249. https://doi.org/10.1016/j.fuel.2019.03.006
- Farahati R, Mousavi-Khoshdel SM, Ghaffarinejad A, Behzadi H (2020). Experimental and computational study of penicillamine drug and cysteine as water-soluble green corrosion inhibitors of mild steel. Prog Org Coatings, 142: 105567. https://doi.org/10.1016/j.porgcoat.2020.105567
- Tan B, Xiang B, Zhang S, et al (2021). Papaya leaves extract as a novel eco-friendly corrosion inhibitor for Cu in H2SO4 medium. J Colloid Interface Sci, 582: 918–931. https://doi.org/10.1016/j.jcis.2020.08.093
- Al-Otaibi MS, Al-Mayouf AM, Khan M, et al (2014). Corrosion inhibitory action of some plant extracts on the corrosion of mild steel in acidic media. Arab J Chem, 7: 340–346. https://doi.org/10.1016/j.arabjc.2012.01.015
- Buchweishaija J (2009). Phytochemicals as green corrosion inhibitors in various corrosive media: a review. Tanzania J Sci, 35:
- Al-Akhras N, Mashaqbeh Y (2021). Potential use of eucalyptus leaves as green corrosion inhibitor of steel reinforcement. J Build Eng, 35: 101848. https://doi.org/10.1016/j.jobe.2020.101848
- Liao LL, Mo S, Luo HQ, Li NB (2018). Corrosion protection for mild steel by extract from the waste of lychee fruit in HCl solution: Experimental and theoretical studies. J Colloid Interface Sci, 520: 41–49. https://doi.org/10.1016/j.jcis.2018.02.071
- Hassannejad H, Barati A, Nouri A (2019). The use of nanoemulsion-based strategies to improve corrosion inhibition efficiency of Thyme-based inhibitor. J Mol Liq, 296: 112110. https://doi.org/10.1016/j.molliq.2019.112110
- Oguzie EE, Onuchukwu AI, Okafor PC, Ebenso EE (2006). Corrosion inhibition and adsorption behaviour of Ocimum basilicum extract on aluminium. Pigment Resin Technol, 35: 63–70. https://doi.org/10.1108/03699420610652340
- Noor EA (2009). Potential of aqueous extract of Hibiscus sabdariffa leaves for inhibiting the corrosion of aluminum in alkaline solutions. J Appl Electrochem, 39: 1465–1475. https://doi.org/10.1007/s10800-009-9826-1
- Abd Aziz NA, Hasham R, Sarmidi MR, et al (2021). A review on extraction techniques and therapeutic value of polar bioactives from Asian medicinal herbs: Case study on Orthosiphon aristatus, Eurycoma longifolia and Andrographis paniculata. Saudi Pharm J, 29: 143–165. https://doi.org/10.1016/j.jsps.2020.12.016
- Xhanari K, Finšgar M, Knez Hrnčič M, et al (2017). Green corrosion inhibitors for aluminium and its alloys: a review. RSC Adv, 7: 27299–27330. https://doi.org/10.1039/C7RA03944A
- Chua LS, Latiff NA, Mohamad M (2016). Reflux extraction and cleanup process by column chromatography for high yield of andrographolide enriched extract. J Appl Res Med Aromat Plants, 3: 64–70. https://doi.org/10.1016/j.jarmap.2016.01.004
- Ahmed I, Gadir S, Elgilany E, Abdallah T (2016). Commiphora africana Resin Phytochemical Analysis & Some Biological Aspects. European J Med Plants, 13: 1–11. https://doi.org/10.9734/EJMP/2016/22531
- Qin D, Xi J (2021). Flash extraction: An ultra-rapid technique for acquiring bioactive compounds from plant materials. Trends Food Sci Technol, 112: 581–591. https://doi.org/10.1016/j.tifs.2021.04.025
- Abubakar A, Haque M (2020). Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J Pharm Bioallied Sci, 12: 1. https://doi.org/10.4103/jpbs.JPBS_175_19
- Xhanari K, Finšgar M (2019). Organic corrosion inhibitors for aluminum and its alloys in chloride and alkaline solutions: A review. Arab J Chem, 12: 4646–4663. https://doi.org/10.1016/j.arabjc.2016.08.009
- Zhang K, Yang W, Xu B, et al (2018). Inhibitory effect of konjac glucomanan on pitting corrosion of AA5052 aluminium alloy in NaCl solution. J Colloid Interface Sci, 517: 52–60. https://doi.org/10.1016/j.jcis.2018.01.092
- Ezuber H, El-Houd A, El-Shawesh F (2008). A study on the corrosion behavior of aluminum alloys in seawater. Mater Des, 29: 801–805. https://doi.org/10.1016/j.matdes.2007.01.021
- Halambek J, Berković K, Vorkapić-Furač J (2013). Laurus nobilis L. oil as green corrosion inhibitor for aluminium and AA5754 aluminium alloy in 3% NaCl solution. Mater Chem Phys, 137: 788–795. https://doi.org/10.1016/j.matchemphys.2012.09.066
- Hajsafari N, Razaghi Z, Tabaian SH (2021). Electrochemical study and molecular dynamics (MD) simulation of aluminum in the presence of garlic extract as a green inhibitor. J Mol Liq, 336: 116386. https://doi.org/10.1016/j.molliq.2021.116386
- Abdel-Gaber AM, Khamis E, Abo-ElDahab H, Adeel S (2008). Inhibition of aluminium corrosion in alkaline solutions using natural compound. Mater Chem Phys, 109: 297–305. https://doi.org/10.1016/j.matchemphys.2007.11.038
- Nik WBW, Zulkifli F, Sulaiman O, et al (2012). Study of Henna ( Lawsonia inermis ) as Natural Corrosion Inhibitor for Aluminum Alloy in Seawater. IOP Conf Ser Mater Sci Eng, 36: 012043. https://doi.org/10.1088/1757-899X/36/1/012043
- Nik WBW, Zulkifli F, Rosliza R, Rahman MM (2011). Lawsonia Inermis as green inhibitor for corrosion protection of aluminium alloy. Int J Mod Eng Res Technol, 1: 723–728
- Udensi SC, Ekpe OE, Nnanna LA (2021). Corrosion inhibition performance of low cost and eco-friendly Treculia africana leaves extract on aluminium alloy AA7075-T7351 in 2.86% NaCl solutions. Sci African, 12: e00791. https://doi.org/10.1016/j.sciaf.2021.e00791
- Zulkifli F, Ali N, Yusof MSM, et al (2017). The Effect of Concentration of Lawsonia inermis as a Corrosion Inhibitor for Aluminum Alloy in Seawater. Adv Phys Chem, 2017: 1–12. https://doi.org/10.1155/2017/8521623
- Olakolegan OD, Owoeye SS, Oladimeji EA, Sanya OT (2020). Green synthesis of Terminalia Glaucescens Planch (Udi plant roots) extracts as green inhibitor for aluminum (6063) alloy in acidic and marine environment. J King Saud Univ - Sci, 32: 1278–1285. https://doi.org/10.1016/j.jksus.2019.11.010
- Singh A, Lin Y, Liu W, et al (2014). Plant derived cationic dye as an effective corrosion inhibitor for 7075 aluminum alloy in 3.5% NaCl solution. J Ind Eng Chem, 20: 4276–4285. https://doi.org/10.1016/j.jiec.2014.01.033
- Fernine Y, Ech-chihbi E, Arrousse N, et al (2021). Ocimum basilicium seeds extract as an environmentally friendly antioxidant and corrosion inhibitor for aluminium alloy 2024 -T3 corrosion in 3 wt% NaCl medium. Colloids Surfaces A Physicochem Eng Asp, 627: 127232. https://doi.org/10.1016/j.colsurfa.2021.127232
- Radi M, Melian R, Galai M, et al (2021). Pumpkin seeds as an eco-friendly corrosion inhibitor for 7075-T6 alloy in 3.5% NaCl solution: Electrochemical, surface and computational studies. J Mol Liq, 337: 116547. https://doi.org/10.1016/j.molliq.2021.116547
- Gerengi H (2012). Anticorrosive Properties of Date Palm ( Phoenix dactylifera L.) Fruit Juice on 7075 Type Aluminum Alloy in 3.5% NaCl Solution. Ind Eng Chem Res, 51: 12835–12843. https://doi.org/10.1021/ie301771u
- Elgahawi H, Gobara M, Baraka A, Elthalabawy W (2017). Eco-Friendly Corrosion Inhibition of AA2024 in 3.5% NaCl Using the Extract of Linum usitatissimum Seeds. J Bio- Tribo-Corrosion, 3: 55. https://doi.org/10.1007/s40735-017-0116-x
- Nnanna LA, Owate IO, Nwadiuko OC, et al (2013). Adsorption and Corrosion Inhibtion of Gnetum Africana Leaves Extract on Carbon Steel. Int J Mater Chem. https://doi.org/10.5923/j.ijmc.20130301.03
- Shehata OS, Korshed LA, Attia A (2018). Green Corrosion Inhibitors, Past, Present, and Future. In: Corrosion Inhibitors, Principles and Recent Applications. InTech
- Montemor MF (2016). Fostering Green Inhibitors for Corrosion Prevention. In: Springer Series in Materials Science. pp 107–137
- Abdelaziz S, Benamira M, Messaadia L, et al (2021). Green corrosion inhibition of mild steel in HCl medium using leaves extract of Arbutus unedo L. plant: An experimental and computational approach. Colloids Surfaces A Physicochem Eng Asp, 619: 126496. https://doi.org/10.1016/j.colsurfa.2021.126496
- Nnanna L a, Onwuagba BN, Mejeha IM, Okeoma KB (2010). Inhibition effects of some plant extracts on the acid corrosion of aluminium alloy. African J pure Appl Chem
- Badiea AM, Mohana KN (2009). Corrosion Mechanism of Low-Carbon Steel in Industrial Water and Adsorption Thermodynamics in the Presence of Some Plant Extracts. J Mater Eng Perform, 18: 1264–1271. https://doi.org/10.1007/s11665-009-9378-x
- Ogunleye OO, Arinkoola AO, Eletta OA, et al (2020). Green corrosion inhibition and adsorption characteristics of Luffa cylindrica leaf extract on mild steel in hydrochloric acid environment. Heliyon, 6: e03205. https://doi.org/10.1016/j.heliyon.2020.e03205
- Wei H, Heidarshenas B, Zhou L, et al (2020). Green inhibitors for steel corrosion in acidic environment: state of art. Mater Today Sustain, 10: 100044. https://doi.org/10.1016/j.mtsust.2020.100044
- Chadili M, Rguiti MM, El Ibrahimi B, et al (2021). Corrosion Inhibition of 3003 Aluminum Alloy in Molar Hydrochloric Acid Solution by Olive Oil Mill Liquid By-Product. Int J Corros, 2021: 1–13. https://doi.org/10.1155/2021/6662395
- Patiha, Heraldy E, Hidayat Y, Firdaus M (2016). The langmuir isotherm adsorption equation: The monolayer approach. IOP Conf Ser Mater Sci Eng, 107: 012067. https://doi.org/10.1088/1757-899X/107/1/012067
- Nethaji S, Sivasamy A, Mandal AB (2013). Adsorption isotherms, kinetics and mechanism for the adsorption of cationic and anionic dyes onto carbonaceous particles prepared from Juglans regia shell biomass. Int J Environ Sci Technol, 10: 231–242. https://doi.org/10.1007/s13762-012-0112-0
- Honarvar Nazari M, Shihab MS, Havens EA, Shi X (2020). Mechanism of corrosion protection in chloride solution by an apple-based green inhibitor: experimental and theoretical studies. J Infrastruct Preserv Resil, 1: 7. https://doi.org/10.1186/s43065-020-00007-w
- El-Latif MMA, Ibrahim AM, El-Kady MF (2010). Adsorption equilibrium, kinetics and thermodynamics of methylene blue from aqueous solutions using biopolymer oak sawdust composite. J Am Sci
- SHU Q, LIAO C, ZOU W, et al (2021). Recovery of rare earth element ytterbium(III) by dried powdered biomass of spirulina: Adsorption isotherm, kinetic and thermodynamic study. Trans Nonferrous Met Soc China, 31: 1127–1139. https://doi.org/10.1016/S1003-6326(21)65566-8
- Wang J, Guo X (2020). Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere, 258: 127279. https://doi.org/10.1016/j.chemosphere.2020.127279
- Mittal A, Kurup L, Mittal J (2007). Freundlich and Langmuir adsorption isotherms and kinetics for the removal of Tartrazine from aqueous solutions using hen feathers. J Hazard Mater, 146: 243–248. https://doi.org/10.1016/j.jhazmat.2006.12.012
- Ndi Nsami J, Ketcha Mbadcam J (2013). The Adsorption Efficiency of Chemically Prepared Activated Carbon from Cola Nut Shells by on Methylene Blue. J Chem, 2013: 1–7. https://doi.org/10.1155/2013/469170
- Ayawei N, Ebelegi AN, Wankasi D (2017). Modelling and Interpretation of Adsorption Isotherms. J Chem, 2017: 1–11. https://doi.org/10.1155/2017/3039817
- Brdar M, Šćiban M, Takači A, Došenović T (2012). Comparison of two and three parameters adsorption isotherm for Cr(VI) onto Kraft lignin. Chem Eng J, 183: 108–111. https://doi.org/10.1016/j.cej.2011.12.036