Vol 62 No 2 (2017): Journal of the Chilean Chemical Society
Original Research Papers
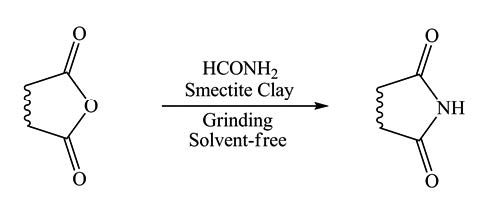
GRINDING IMIDATION OF ANHYDRIDES ON SMECTITE CLAYS AS RECYCLABLE AND HETEROGENEOUS CATALYSTS UNDER SOLVENT-FREE CONDITIONS
Published
June 16, 2017
Keywords
- Smectite clays,
- Grindstone technique,
- Solvent-free,
- Imidation
How to Cite
Marvi, O. (2017). GRINDING IMIDATION OF ANHYDRIDES ON SMECTITE CLAYS AS RECYCLABLE AND HETEROGENEOUS CATALYSTS UNDER SOLVENT-FREE CONDITIONS. Journal of the Chilean Chemical Society, 62(2). Retrieved from https://jcchems.com/index.php/JCCHEMS/article/view/194
Copyright (c) 2017 Omid Marvi

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
Imidation of various anhydrides employing solvent-free grindstone technique using smectite clays as recyclable and green catalysts was examined and obtained excellent yields.
References
- a) S. K. Upadhyay, S. R. K. Pingali , B. S. Jursic, Tetrahedron Lett. 51, 2215, (2010); b) E. Benjamin, Y. Hijji, Molecules, 13, 157, (2008); c) B. Guthrie, Z. Wang, J. Li, Mater. Res. Soc. Symp. Proc., 1091, (2008).
- a) J. O. Osby, M. G. Martin, B. Ganem, Tetrahedron Lett. 25, 2093, (1984); b) P. G. M. Wuts, T. W. Greene, Greene’s Protective Groups in Organic Synthesis, 4th ed.; Wiley: New York, (2007).
- a) S. M. Sondhi, R. Rani, P. Roy, S. K. Agrawal, A. K. Saxena, Bioorg. Med. Chem. Lett., 19, 1534, (2009); b) S. G. Stewart, M. E. Polomska, R. W. Lim, Tetrahedron Lett., 48, 2241, (2007).
- a) K. Kafi, D. J. Betting, R. E. Yamada, M. Bacica, K. K. Steward, Timmerman, J. M. Mol. Immunol., 448, (2009); b) S. Karim, C. S. Johansson, J. K. Weltman, Nucleic Acids Res., 23, 2037, (1995); c) R. Wattanadilok, P. Sawangwong, C. Rodrigues, H. Cidade, M. Pinto, E. Pinto, A. Silva, A. Kijjoa, Mar. Drugs, 5, 40, (2007).
- O. H. Wheeler, O. Rosado, In The Chemistry of Amides, J. Zabicky, Ed., John Wiley and Sons: New York, 335, (1970).
- R. B. Bates, F. A. Fletcher, K. D. Janda, W. A. Miller, J. Org. Chem., 49, 3038, (1984).
- A. Schnyder, F., Indolese, J. Org. Chem., 67, 594, (2002).
- M. B. Andrus, W. Li, R. F. Keyes, Tetrahedron Lett., 39, 5465, (1998).
- F. Wang, H. Liu, H. Fu, Y. Jiang, Y. Zhao, Adv. Synth. Catal., 351, 246, (2009).
- L. Wang, H. Fu, Y. Jiang, Y. Zhao, Chem. Eur. J., 14, 10722, (2008).
- a) L. Xu, S. Zhang, M. L. Trudell, Chem. Commun., 1668, (2004); b) Z. Jin, B. Xu, G. B. Hammond, Tetrahedron Lett., 52, 1956, (2011); c) M. M. Khodaei, E. Nazari, Tetrahedron Lett., 53, 2881, (2012).
- a) T. Cseri, S. Bekassy, F. Figueras, E. Cseke, E., de Menorval, R. Dutartre, Appl. Catal. A, 132, 141, (1995); b) T-K. Huang, R. Wang, L. Shi, X-X. Lu , Catal. Commun., 9, 1143, (2008).
- a) M. Choudary, N. S. Chowdari, M. L. Kantam, R. Kannan, Tetrahedron Lett., 40, 2859, (1999). b) P. R. Crisostomo, R. Carrillo, T. Martin, V. S. Martin, Tetrahedron Lett., 46, 2829, (2005).
- a) M. D. Nikalje, P. Phukan, A. Sudalai, Org. Prep. Proceed. Int., 32, 1, (2000); b) T. Kawabata, T. Mizugaki, K. Ebitani, K. Kaneda, Tetrahedron Lett., 44, 9205, (2003).
- a) P. Lasszlo, Science, 235, 1473, (1987); b) B. Baghernejad, Lett. Org. Chem., 7, 255, (2010); c) S. Agarwal, J. N. Ganguli, J. Mol. Catal. A: Chem., 372, 44, (2013).
- a) O. Marvi, M. Giahi, Bull. Korean Chem. Soc., 30, 2918, (2009); b) D. Habibi, O. Marvi, Catal. Commun., 8, 127, (2007); c) O. Marvi, M. Nikpasand, Lett. Org. Chem., 10, 353, (2013).
- a) Determined in our laboratory by temperature-programmed desorption of ammonia gas (NH3-TPD): P. Berteau, B. Delmon, Catal. Today, 5, 121, (1989); b) Determined in our laboratory by the BET method: S. Brunauer, P. H. Emmett, E. Teller, J. Am. Chem. Soc., 60, 309, (1938).
- a) R. Ballini, G. Bosica, R. Maggi, M. Ricciutelli, P. Righi, G. Sartori, R. Sartorio, Green Chemistry, 3, 178, (2001); b) G. Sartori, F. Bigi, R. Maggi, A. Mazzacani, G. Oppici, Eur. J. Org. Chem., 2513, (2001).
- a) K. Kacprzak, Synth. Commun., 33, 1499, (2003). b) G. Hamprecht, J. Varwig, W. Rohr, US4680412 A, July 14, (1987).
- a) R. A. W. N. Filho, M. A. T. Palm-Forster, R. N. de Oliveira, Synth. Commun., 43, 1571, (2013); b) E. Benjamin, Y. Hijji, Molecules, 13, 157, (2008); c) Y. Peng, G. Song, X. Qian, Synth. Commun., 31(12), 1927, (2001).


