MAGNETIC SOLID PHASE EXTRACTION FOR A NEW HPLC METHOD FOR THE DETERMINATION OF GEMIFLOXACIN IN HUMAN PLASMA AND BREAST MILK
- HPLC,
- Gemifloxacin,
- Magnetic solid phase extraction,
- Plasma,
- Breast milk
- UV detection ...More
Copyright (c) 2017 Zehra Durmus, SerIfe EvrIm KepekcI Tekkeli, Mustafa Volkan KIzIltas, Armagan Onal

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
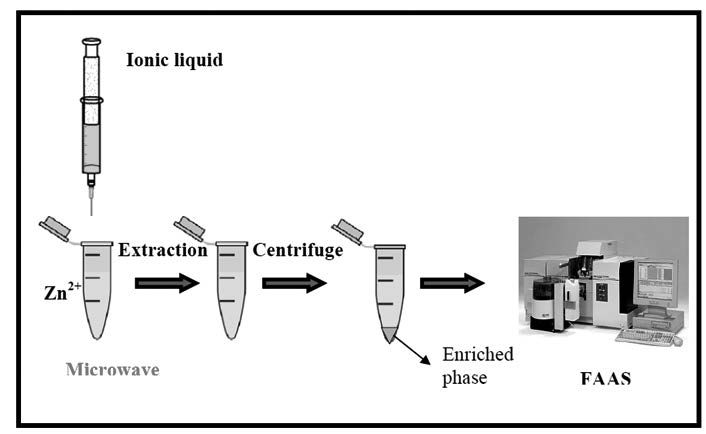
A simple analytical procedure, magnetic solid phase extraction combination to HPLC, has been developed for the analysis of gemifloxacin in human plasma and breast milk. Before chromatographic separation, magnetic solid phase extraction has been applied for sample preparation which is currently preffered extraction technique accordingly its simple, fast and efficient procedure. Fe3O4 magnetic nanoparticles have been used as magnetic adsorbents, the adsorption process has been optimized. RP C18 column has been used with mobile phase composed of acetonitrile-10 mM orthophosphoric acid including 1 mL/L triethylamine (60:40) by isocratic elution with flow rate of 1.3 mL/min. Calibration curve is linear over the range of 0.5-30 μg/mL and 0.5-20 μg/mL for plasma and breast milk, respectively. LOD and LOQ has been found to be 0.15 and 0.5μg/mL for both matrices. Intraday and interday RSD values are less than 3.57% for both assays.
Moreover, the newly developed method provides fast, simple, cost reduced and sensitive assay for gemifloxacin.
References
- A. N. Gurpinar, E. Balkan, N. Kilic, I. Kiristioglu and H. Doğruyol, J. Int. Med. Res. 25 (1997), 302.
- P. M. Fleiss, J. Hum. Lact. 8(1) (1992), 7.
- J. K. Aronson, Fluoroquinolones Meyler’s Side Effects of Drugs: The International Encyclopedia of Adverse Drug Reactions and Interactions, fifteenth ed., Elsevier, Amsterdam, 2006.
- M. N. Lowe, H. M. Lamb, Drugs. 59 (2000), 1137.
- S. Malgorzata, K. Rafal, S. Jacek, J. Marek and B. Boguslaw, J. Chromatogr. A, 1272 (2013), 41.
- A. R. Rote, S. P. Pingle, J. Chromatogr. B, 877 (29) (2009), 3719.
- W. M. El-Koussi, N. N. Atia, A. M. Mahmoud and S. R. El-Shabouri, J. Chromatogr. B, 967, (2014), 98.
- E. Doyle, S. E. Fowles, D. F. McDonnell, S. A. White, J. Chromatogr. B, 746 (2000), 191.
- B. M. H. Al-Hadiya, A. A. Khady, G. A. E. Mostafa, Talanta, 83 (1) (2010), 110.
- O. Sagirli, S. Demirci and A. Onal, Luminescence, DOI 10.1002/bio.2901 (2015).
- C. F. Poole, Trends Analyt. Chem. 22 (2003), 362.
- M. Wierucka and M. M. Biziuk, Trends in Analyt. Chem. 59 (2014), 50.
- R. Karami-Osboo, R. Miri, K. Javidnia, M. H Shojaee, and F. Kobarfard, Anal. Methods, 7 (2015), 1586.
- E. Behnam, Y. Yadollah, S. Shahram and T. Mohammad, Anal. Chim. Acta, 885, 98, (2015).
- K. O. Rouhollah, M. Ramin, J. Katayoun, H. S.Mohammad and K. Farzad, Anal. Methods, 7, 1586, (2015).
- Y. B. Luo, Z. G. Shi, Q. Gao and Y. Q. Feng, J. Chromatogr. A, 1218, 1353, (2011).
- A. V. Herrera-Herrera, J.Hernández-Borges, M. M. Afonso, J. A. Palenzuela and M. Á. Rodríguez-Delgado, Talanta 116, 695, (2013).
- A. Z. Hossein, T. Zeynab, Talanta 134 (1), 387, (2015).
- M. Tang, Q. Wang, M. Jiang, L. Xu, Z. G. Shi, T. Zhang and Y. Liu, Talanta 130 (1), 427, (2014).
- Y. Yadollah, F. Mohammad, J. Pharm. Anal., 4 (4), 279, (2014).
- K. C. de Souza, G. F. Andrade, I. Vasconcelos, I. M. de Oliveira Viana, C. Fernandes and E.M. de Sousa, Mater. Sci. Eng. C Mater. Biol. Appl. 40 (1) 275, (2014).
- A. A. Ali, K. Sara, E. Homeira, S. Nafiseh and J. Niloofar, Int. J. Pharm. 494 (1), 102, (2015).
- J. Mürbe, A. Rechtenbach and T. Töpfer, Mat. Chem. Phys. 110, 426, (2008).
- A. P. Khandhar, R. M. Ferguson and K. M. Krishnan, J. Appl. Phys. 109 (7), 310, (2011).
- The International Conference on Harmonisation (ICH), ICH Technical Requirements for Registration of Pharmaceuticals for Human Use on validation of analytical procedures Q2A. IFPM, 2005, Geneva.


