DETERMINATION OF BRACHIARIA SPP. FORAGE QUALITY BY NEAR-INFRARED SPECTROSCOPY AND PARTIAL LEAST SQUARES REGRESSION
- Forage,
- NIRS,
- Partial least squares,
- Chemical properties
Copyright (c) 2017 Mariel Monrroy, Dehylis Gutiérrez, Marissa Miranda, Karla Hernández, José Renán GarcÍa

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
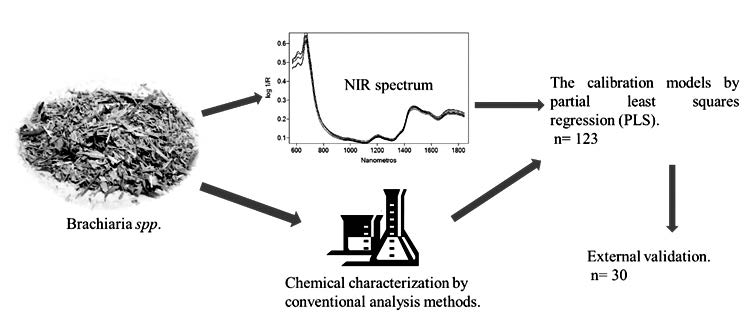
Characterizing the chemical properties of forage is critical for the production of improved pastures and livestock development. Conventional analysis methods are very time- and material-consuming, whereas near-infrared spectroscopy (NIRS) and chemometric analyses allow a fast simultaneous determination of various chemical or physical properties without the use of solvents or large sample amounts. The present research involved the development of models based on NIRS and partial least squares regression (PLS) to estimate the neutral detergent fiber (NDF), acid detergent fiber (ADF), cellulose, and crude protein (CP) contents in
Brachiaria spp. forage samples. The models were constructed using spectral data in the range of 800 to 1850 nm. Different preprocessing methods were applied, such as standard normal variate and first-/second-derivative transformations. The obtained calibration models were internally cross-validated, displaying validation errors similar to those obtained for conventional methods. The predictive abilities of the developed models were evaluated for external set samples. NDF, ADF, cellulose, and CP contents were estimated with relative errors of prediction (REPs) of 1.8, 2.6, 4.1, and 8.5%, respectively. NIRS predictions are a useful and profitable tool for fast multi-sample chemical property analysis that is required for the assessment of forage quality. The obtained models are suitable for estimating the key chemical characteristics of forage quality. This research contributes a new approach to determining the quality of Brachiaria spp. forage and provides a new technological tool for the improvement of this crop.
References
- H. Zaker, H. Aminpanah, H. Darkhal, Agriculture and Forestry 60, 193, (2014).
- F. Amiri, A. Shariff, M. Rashid, Songklanakarin J. Sci. Technol. 34, 577 (2012).
- A. F. Ribeiro, J. D. Messana, P. H. M. Dian, R. A. Reis, A. C. Ruggieri, E. B. Malheiros, T. T. Berchielli, Ital. J. Anim. Sci. 13, 36, (2014).
- J. Stuth, A. Jama, D. Tolleson, Field Crop Res. 84, 45, (2003).
- V. Bellon, J. L. Vigneau, F. Sévila, Food Control 5, 21, (1994).
- H. Huang, H. Yu, H. Xu, Y. Ying, J. Food Eng. 87, 303, (2008).
- H. W. Siesler, Y. Ozaki, S. Kawata, H. M. Heise Near-Infrared Spectroscopy: Principles, Instruments, Applications, 1st ed. Wiley-VCH, Weinheim, 2002.
- Y. Sun, L. Lin, H. Deng, J. Li, B. He, R. Sun, P. Ouyang, BioResources 3, 297, (2008).
- Z.-H. Jiang, Z. Yang, C.-L. So, C.-Y. Hse, J. Wood Sci. 53, 449, (2007).
- M. St. Luce, N. Ziadi, B. J. Zebarth, C. A. Grant, G. F. Tremblay, E. G. Gregorich, Geoderma 232, 449, (2014).
- A. Salgó, S. Gergely, J. Cereal Sci. 56, 31, (2012).
- M. Monrroy, R. T. Mendonça, J. Ruiz, J. Baeza, J. Freer, J. Wood Chem. Technol. 29, 150, (2009).
- R. Castillo, J. Baeza, J. Rubilar, Á. Rivera, J. Freer, Appl. Biochem. Biotechnol. 168, 2028, (2012).
- M. Monrroy, J. R. Garcia, E. Troncoso, J. Freer, J. Chem. Technol. Biot. 90, 1281, (2015).
- Y.-H. Jia, X.-P. Liu, Y.-C. Feng, C.-Q. Hu, AAPS PharmSciTech 12, 738, (2011).
- S. Landau, T. Glasser, L. Dvash, Small Ruminant Res. 61, 1, (2006).
- J. J. Baloyi, H. Hamudikuwanda, N. Berardo, M. Ordoardi, N. T. Ngongoni, Afr. J. Agric. Res. 8, 868, (2013).
- A. M. De Souza-Kaneshima, C. Simioni, M. F. Felismino, A. B. Mendes- Bonato, C. Risso-Pascotto, C. Pessim, M. S. Pagliarini, C. B. Do Valle, Plant Breeding 129, 186, (2010).
- E. R. Canchila, M. Soca, F. Ojeda, R. Machado, N. Canchila, Pastos y Forrajes 33, 7, (2010).
- AOAC, in Official Methods of Analysis (18th), AOAC International, Arlington, VA 2005.
- P. J. Van Soest, J. B. Robertson, B. A. Lewis, J. Dairy Sci. 74, 3583, (1991).
- L. Campo, A. B. Monteagudo, B. Salleres, P. Castro, J. Moreno-Gonzalez, Span. J. Agric. Res. 11, 463 (2013).
- R. Kowsar, G. R. Ghorbani, M. Alikhani, M. Khorvash, A. Nikkhah, J. Dairy Sci. 91, 4755, (2008).
- K. M. Lundberg, P. C. Hoffman, L. M. Bauman, P. Berzaghi, The Professional Animal Scientist 20, 262, (2004).
- P. Castro in Lowland and Grasslands of Europe: Utilization and Development, G. Fisher and B. Frankow-Lindberg eds. Food and Agriculture Organization of the United Nations (FAO), Rome, 2002; pp. 225-22826. G. R. Hodge, W. C. Woodbridge, J. Near Infrared Spec. 18, 367, (2010).
- A. C. Olivieri and G. M. Escandar in Practical Three-Way Calibration, Elsevier, Boston, 2014; pp. 65–92.
- W. Saeys, A. M. Mouazen, H. Ramon, Biosyst. Eng. 91, 393, (2005).
- P. Jones, L. R. Schimleck, G. Peter, R. Daniels, A. Clark, Wood Sci. Technol. 40, 709, (2006).
- S. S. Kelley, T. G. Rials, R. Snell, L. H. Groom, A. Sluiter, Wood Sci. Technol. 38, 257, (2004).
- J. Workman, L. Weyer, Practical Guide to Interpretive Near-Infrared Spectroscopy, Taylor & Francis Group, New York and Florida, 2008.
- Shenk, J. Workman, M. Westerhaus in Handbook of Near-Infrared Analysis, D. Burns, E. Ciurczak eds. CRC Press, New York and Florida, 2008; pp. 34–32.


