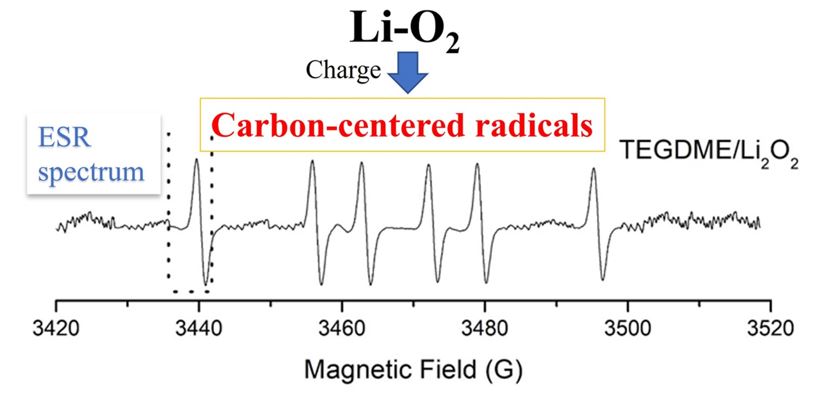
- carbon-centered radicals,
- Li-O2 cell,
- oxygen-centered radicals,
- peroxide of hydrogen,
- peroxide of lithium
- tetraethylene glycol dimethyl ether decomposition ...More
Copyright (c) 2021 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
In this work we studied the decomposition of the TEGDME (tetraethylene glycol dimethyl ether) solvent in conditions simulating the charge of a Li-O2 cell in the presence and absence of peroxide of hydrogen and peroxide of lithium trough ESR studies. We detected the formation of radical species, although in low concentrations, originating from solvent decomposition reactions during the oxidation process, in the absence of peroxides. On the other hand, when introducing H2O2 and H2O into the system, oxygen-centered radical species superoxide and hydroxyl were detected. In addition, in the presence of Li-O2, carbon-centered radical species were detected that clearly show the decomposition of the solvent. Finally, the results show that it is very important that the charging process of a Li-O2 cell is carried out through direct oxidation via 2 e- to Li2O2 to avoid formation of radical species that cause deterioration of the solvent.
References
- X.H. Yao, Q. Dong, Q.M. Cheng, D.W. Wang, Angew. Chem. Int. Ed. 55, 11344-11353, (2016).
- M. Balaish, A. Kraytsberg, Y. Ein-Eli, PCCP, 16, 2801 (2014).
- P. Du, J. Lu, K.C. Lau, X.Y. Luo, J. Bareno, X.Y. Zhang, Y. Ren, Z.C. Zhang, L.A. Curtiss, Y.K. Sun, K. Amine, PCCP, 15 , 5572 (2013).
- E. Nasybulin, W. Xu, M.H. Engelhard, Z. Nie, S.D. Burton, L. Cosimbescu, M.E. Gross, J.-G. Zhang, J. Phys. Chem. C. 117, 2635 (2013).
- M.M.O. Thotiyl, S.A. Freunberger, Z.Q. Peng, P.G. Bruce, J. Am. Chem. Soc. 135, 494 (2013).
- B.D. McCloskey, A. Speidel, R. Scheffler, D.C. Miller, V. Viswanathan, J.S. Hummelshoj, J.K. Norskov, A.C. Luntz, J. Phys. Chem. Lett. 3, 997 (2012).
- J. Read, K. Mutolo, M. Ervin, W. Behl, J. Wolfenstine, A. Driedger, D. Foster, J. Electrochem. Soc. 150, A1351 (2003).
- W. Xu, K. Xu, V.V. Viswanathan, S.A. Towne, J.S. Hardy, J. Xiao, D.H. Hu, D.Y. Wang, J.G. Zhang, J. Power Sources, 196, 9631, (2011)
- S.A. Freunberger, Y. Chen, Z. Peng, J.M. Griffin, L.J. Hardwick, F. Bardé, P. Novák, P.G. Bruce, J. Am. Chem. Soc., 133, 8040 (2011).
- J. Read, J. Electrochem. Soc., 153, A96 (2006).
- R. Younesi, M. Hahlin, M. Treskow, J. Scheers, P. Johansson, K. Edstrom, J. Phys. Chem. C, 116, 18597 (2012).
- W. Xu, J.Z. Hu, M.H. Engelhard, S.A. Towne, J.S. Hardy, J. Xiao, J. Feng, M.Y. Hu, J. Zhang, F. Ding, M.E. Gross, J.G. Zhang, J. Power Sources , 215, 240 (2012).
- H.G. Jung, J. Hassoun, J.B. Park, Y.K. Sun, B. Scrosati, Nat. Chem., 4 , 579 (2012).
- Z. Ma, X.X. Yuan, L. Li, Z.F. Ma, D.P. Wilkinson, L. Zhang, J.J. Zhang, Energy Environ. Sci. 8, 2144 (2015).
- C.O. Laoire, S. Mukerjee, K.M. Abraham, E.J. Plichta, M.A. Hendrickson, J. Phys. Chem. C, 114, 9178 (2010).
- S. Ferrari, E. Quartarone, C. Tomasi, M. Bini, P. Galinetto, M. Fagnoni, P. Mustarelli, J. Electrochem. Soc. 162, A3001 (2015).
- C.O. Laoire, S. Mukerjee, E.J. Plichta, M.A. Hendrickson, K.M. Abraham, J. Electrochem. Soc. 158, A302 (2011).
- B.D. McCloskey, C.M. Burke, J.E. Nichols, S.E. Renfrew, Chem. Commun. 51, 12701 (2015).
- F.S. Gittleson, R.C. Sekol, G. Doubek, M. Linardi, A.D. Taylor, PCCP, 16, 3230 (2014).
- L. Wang, Y. Zhang, Z. Liu, L. Guo, Z. Peng, GEE, 2, 186 (2017).
- M.J. Trahan, I. Gunasekara, S. Mukerjee, E.J. Plichta, M.A. Hendrickson, K.M. Abraham, J. Electrochem. Soc.161, A1706 (2014).
- A. Khetan, H. Pitsch, V. Viswanathan, J. Phys. Chem. Lett. 5, 2419 (2014).
- T. Zhang, R. Amine, X. Bi, Y. Qin, M. Li, S. Al-Hallaj, F. Huo, J. Lu, K. Amine, J. Mater. Chem. A, 7 ,15615 (2019).
- S.Y. Kang, Y.F. Mo, S.P. Ong, G. Ceder, Chem. Mater. 25, 3328 (2013).
- B. Aguilera-Venegas, C. Olea-Azar, V.J. Aran, H. Speisky, Future Med. Chem. 5, 1843 (2013).
- M. Carboni, A.G. Marrani, R. Spezia, S. Brutti, J. Electrochem. Soc. 165, A118 (2018).
- N. Mahne, B. Schafzahl, C. Leypold, M. Leypold, S. Grumm, A. Leitgeb, Gernot A. Strohmeier, M. Wilkening, O. Fontaine, D. Kramer, C. Slugovc, Sergey M. Borisov, Stefan A. Freunberger, Nat. Energy, 2, 17036 (2017).
- J. Wandt, P. Jakes, J. Granwehr, H.A. Gasteiger, R.A. Eichel, Angew. Chem. Int. Ed. 55, 6892 (2016).
- M. Park, H. Sun, H. Lee, J. Lee, J. Cho, Adv. Energy Mater. 2, 780 (2012).
- S.A. Freunberger, Y.H. Chen, N.E. Drewett, L.J. Hardwick, F. Barde, P.G. Bruce, Angew. Chem. Int. Ed. 50, 8609 (2011).
- M.M.O. Thotiyl, S.A. Freunberger, Z.Q. Peng, Y.H. Chen, Z. Liu, P.G. Bruce, Nat. Mater. 12, 1049 (2013).
- T. Ogasawara, A. Debart, M. Holzapfel, P. Novak, P.G. Bruce, J. Am. Chem. Soc. 128,1390 (2006).
- Z. Peng, S.A. Freunberger, L.J. Hardwick, Y. Chen, V. Giordani, F. Barde, P. Novak, D. Graham, J.-M. Tarascon, P.G. Bruce, Angew. Chem. Int. Ed. 50, 6351 (2011).
- Y.-C. Lu, Y. Shao-Horn, J. Phys. Chem. Lett. 4, 93 (2013).
- B.M. Gallant, D.G. Kwabi, R.R. Mitchell, J.G. Zhou, C.V. Thompson, Y. Shao-Horn, Energy Environ. Sci. 6, 2518 (2013).
- J. Yang, D. Zhai, H.-H. Wang, K.C. Lau, J.A. Schlueter, P. Du, D.J. Myers, Y.-K. Sun, L.A. Curtiss, K. Amine, PCCP, 15, 3764 (2013).
- Y. Wang, N.-C. Lai, Y.-R. Lu, Y. Zhou, C.-L. Dong, Y.-C. Lu, Joule, 2, 2364 (2018).
- S. Ganapathy, B.D. Adams, G. Stenou, M.S. Anastasaki, K. Goubitz, X.-F. Miao, L.F. Nazar, M. Wagemaker, J. Am. Chem. Soc. 136, 16335 (2014).
- V. Gutmann, Coord. Chem. Rev. 18, 225 (1976).
- Z. Guo, X. Dong, S. Yuan, Y. Wang, Y. Xia, J. Power Sources, 264, 1 (2014).
- K.U. Schwenke, M. Metzger, T. Restle, M. Piana, H.A. Gasteiger, J. Electrochem. Soc. 162, A573 (2015).
- Y.G. Zhu, Q. Liu, Y. Rong, H. Chen, J. Yang, C. Jia, L.-J. Yu, A. Karton, Y. Ren, X. Xu, S. Adams, Q. Wang, Nat. Commun., 8, 14308 (2017).
- X.W. Gao, Y.H. Chen, L. Johnson, P.G. Bruce, Nat. Mater. 15, 882 (2016).