EFFECT OF TEMPERATURE AND CONTENT OF CHLORURATED SALT OF Cu(II), Mn(II) OR Al(III) ON THE DENSITY OF ACIDIFIED SEAWATER AND WATER MIXES SAMPLES

- Sea water,
- Density,
- Expansibility coefficient,
- heap leaching,
- Copper/Manganese/Aluminum ions
Copyright (c) 2022 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
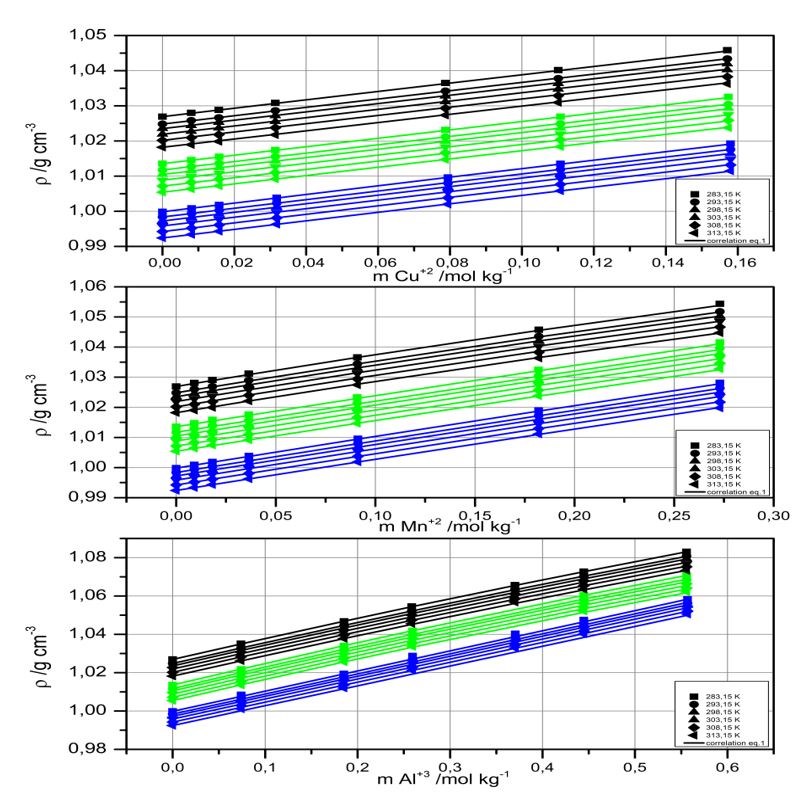
The relationship between the density of seawater and acidified mixtures samples in the presence of up to a maximum concentrations of 0.18 m for Cu(II), 0.34 m for Mn(II) or 0.6 m for Al(III), in selected temperatures used in the copper leaching process (283.15 - 313.15 K), showed a positive linear behavior with salinity and cation concentration.
The influence of temperature on the density rate changes was similar when comparing the three solutes in acidified solutions of different salinity. The effect of temperature on the change in density did not exceed 1% and by concentration, the greatest influence was due to Al(III) with 5.43%, in agreement with its greater relative quantity.
The data obtained, using a vibrating tube density meter, were used to analyze and correlate to the Apelblat equation that was favorably applied to calculate the isobaric expansibility coefficient. The temperature had an influence on the isobaric expansibility coefficient values, suggesting a making interaction effect due to majority anions present in the seawater and mixture samples (Cl- > SO4-2), and a breaking interaction effect when the cations Cu(II), Mn(II) or Al(III) were added.
References
- L.A. Cisternas, E.D. Gálvez, Miner. Process. Extr. Metall. Rev. 39, 18, (2018)
- P.C. Hernández, M.E. Taboada, O.O. Herreros, C.M. Torres, Y. Ghorbani, Hydrometallurgy. 157, 325, (2015)
- W. Chen, S. Yin, A. Wu, L. Wang, X. Chen, Bioresour. Technol. 297, 122453, (2020)
- K.P. Goodboy, T.M. Missimer, Desalin. Water Treat. 194, 1, (2020)
- A. Mahmoudi, S. Shakibania, S. Rezaee, M. Mokmeli, Sep. Purif. Technol. 251, 117394, (2020)
- M. Schlesinger, M. King, K. Sole, W. Davenport, Extractive Metallurgy of Copper, Elsevier, Amsterdam, (2011).
- P. Navarro, C. Vargas, C. Ramírez, Rev. Fac. Ing. 25, 75, (2016)
- C.W. Fetter, Applied hydrology, Waveland Press, Inc.,Illinois, 2001.
- S. hua Yin, L. ming Wang, A. xiang Wu, X. Chen, R. fu Yan, Int. J. Miner. Metall. Mater. 26, 1337, ( 2019)
- M. qing Huang, A. xiang Wu, J. Cent. South Univ. 27, 1432 , (2020)
- I.M. Abdulagatov, L.A. Akhmedova-Azizova, R.M. Aliev, G.B. Badavov, Appl. Geochemistry. 69, 28, (2016)
- A. Apelblat, J. Mol. Liq. 219, 313, (2016)
- L. Hnedkovsky, Y. Kianinia, G. Hefter, J. Chem. Eng. Data. 63, 3860, (2018)
- B. Hu, L. Hnedkovsky, W. Li, G. Hefter, J. Chem. Eng. Data. 61, 1388 (2016)
- M.S. Páez, J.J. Lafont, A. Alvis, Inf. Tecnol. 20, 47, (2009)
- M. Żarska, M. Dzida, A. Apelblat, J. Mol. Liq. 228, 91, (2017)
- P. Venkatesu, M.J. Lee, H.M. Lin, J. Chem. Thermodyn. 39, 1206, ( 2007)
- W. Alavia, J.A. Lovera, T.A. Graber, D. Azúa, I. Soto, Fluid Phase Equilib. 532, 112864 , (2021)
- H. Hou, W. Cui, J. Chen, L. Meng, Y. Guo, T. Deng, J. Chem. 2020, 1, (2020)
- D. Zezin, T. Driesner, C. Sanchez-Valle, J. Chem. Eng. Data. 60, 1181, (2015)
- D. Rowland, P.M. May, J. Solution Chem. 47, 107, (2018)
- Y. Li, Y.H. Li, F.A. Wang, B.Z. Ren, Thermochim. Acta. 568, 189, (2013)
- P.C. Hernández, H.R. Galleguillos, T.A. Graber, E.K. Flores, M.E. Taboada, J. Chem. Eng. Data. 57, 2430, (2012)
- M. Huque, I.A. Siddiquey, M.N. Uddin, J. Chem. Thermodyn. 38, 1474, (2006)
- J. Krakowiak, J. Wawer, J. Chem. Thermodyn. 90, 232, (2015)
- M. Kumar, S. Kant, D. Kaushal, Volumetric, Zeitschrift Fur Phys. Chemie. 233, 255, (2019)
- A. V. Morozov, A. V. Pityk, A.R. Sahipgareev, A.S. Shlepkin, J. Phys. Conf. Ser. 1128, 12103, (2018).
- M. Arrad, M. Kaddami, H. Sippola, P. Taskinen, J. Chem. Eng. Data. 60, 856, (2015)
- N. Boukhalfa, A.H. Méniai, Int. J. Hydrogen Energy. 43, 5358, (2018)
- X. Ji, S.P. Tan, H. Adidharma, M. Radosz, AIChE Annu. Meet. Conf. Proc. 2005, 4442 (2005)
- S.P. Tan, X. Ji, H. Adidharma, M. Radosz, J. Phys. Chem. B. 110, 16694, (2006)
- R. Romeo, P.A. Giuliano Albo, S. Lago, Deep. Res. Part I Oceanogr. Res. Pap. 154, 103157, (2019)
- R. Feistel, A new extended Gibbs thermodynamic potential of seawater, Prog. Oceanogr. 58, 43,(2003)
- F. J. Millero, in The Sea, Vol. 5. Marine Chemistry, A. E. Maxwell et al., John Wiley and Sons eds., New York, 1974; pp. 3-80.
- F.J. Millero, R. Feistel, D.G. Wright, T.J. McDougall, Deep. Res. Part I Oceanogr. Res. Pap. 55, 50, (2008)
- IOC SCOR, IAPSO: The international thermodynamic equation of seawater–2010: Calculation and use of thermodynamic properties , UNESCO(English), París, 2010
- J. García-Abdeslem, J. Appl. Geophys. 183, 104200, ( 2020)
- F.J. Millero, Chemical oceanography, CRC press, Boca Raton, 1996.
- R.A. Bobadilla-Fazzini, A. Pérez, V. Gautier, H. Jordan, P. Parada, Hydrometallurgy. 168, 26, (2017)
- Q. Liu, M. Chen, Y. Yang, Electrochim. Acta. 253, 257, (2017)
- L. Wang, H. Li, Q. Liu, L. Xu, L. Zha, S. Lin, Minerals. 10, 1, (2020)
- D. González-Salgado, J. Troncoso, E. Lomba, Fluid Phase Equilib. 521, 112703, (2020).
- J.P. Hershey, R. Damesceno, F.J. Millero, J. Solution Chem. 13, 825, (1984)
- M.H. Sharqawy, J.H. Lienhard V, S.M. Zubair, Desalin. Water Treat. 29, 355, (2011)
- T.M. Herrington, M.G. Roffey, D.P. Smith, J. Chem. Eng. Data. 31, 221, (1986)
- R. Pogue, G. Atkinson, J. Chem. Eng. Data. 33, 370, (1988)
- R.F. Pogue, G. Atkinson, J. Chem. Eng. Data. 34, 227, (1989)
- A. Apelblat, Citric Acid, Springer, Heidelberg, 2014.
- R.A. Robinson, R.H. Stokes, Electrolyte Solutions, Butterworths, London, 1965.
- Y. Marcus, Chem. Rev. 109, 1346, (2009)