BIOACTIVE METAL COMPLEXES OF Cu(II), Ni(II), AND Zn(II) IONS CONTAINING N-ISONICOTINAMIDO-THIOPHENE-2-CARBALDIMINE SCHIFF BASE AND 1, 10-PHENANTHROLINE: SYNTHESIS, CHARACTERIZATION, ANTIMICROBIAL AND ANTIOXIDANT PROPERTIES

- Antioxidant properties,
- Transition metal complexes
Copyright (c) 2021 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
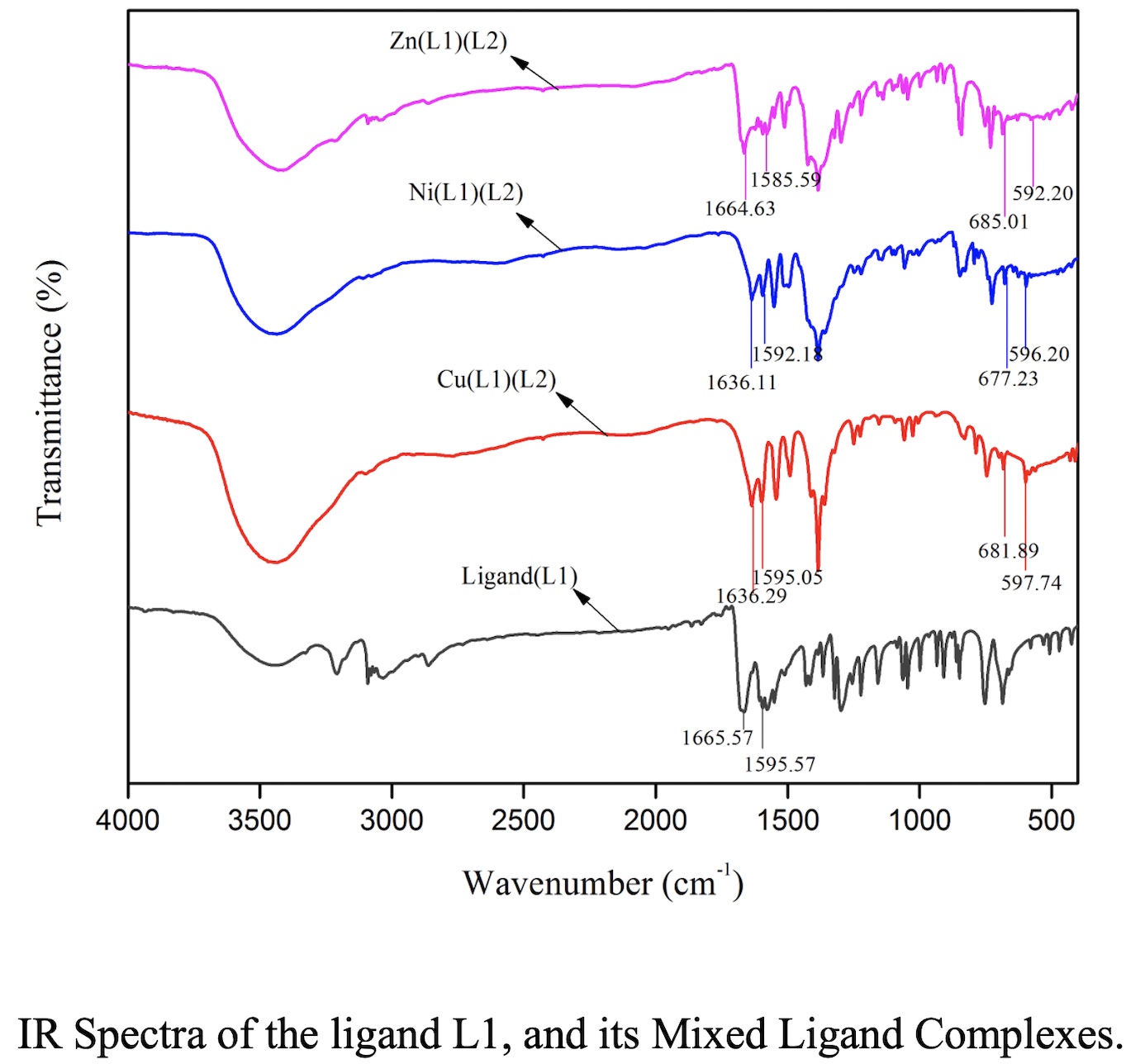
A series of transition metal complexes having general formula [M(Lb)(L1)](NO3)2, (where, M = Cu(II), Ni(II), and Zn(II)) of mixed ligands, Lb = N-Isonicotinamido-Thiophene-2-Carbaldimine, L1 = 1, 10-phenanthroline, were synthesized. The obtained compounds were successfully characterized by different spectroscopic techniques. Conductivity measurements indicated that all complexes were 1:2 electrolytes in nature. IR spectra indicated that ligands coordinated to metals via the carbonyl oxygen atom and the azomethine nitrogen atom. Magnetic moment values and UV-Visible spectra confirmed square planar structure around the Cu(II), and Ni(II) ions, and a tetrahedral geometry around the Zn(II) ion. The metal complexes of Lb and L1 were tested against Escherichia coli and Pseudomonas sp. Among the tested compounds, Zn(II)complex showed higher antibacterial activity over both bacterial strains against reference drug Kanamycin. Moreover, synthesized metal complexes exhibited moderate antioxidant activity than the Schiff base. Cu(II) complexes were found to be most active whereas, Zn(II) complexes showed the lowest antioxidant activity comparable to the BHT.
References
- O.G. Idemudia, E.C. Crystals, “Spectroscopy, Crystal and Molecular Structures of New 4-Acylpyrazolone Dinitrophenylhydrazones”. Crystals, 2016; 6: 127
- G. Naganagowda, R. Meijboom, A. Petsom, “Synthesis and Antimicrobial Activity of New Schiff Base Compounds Containing 2-Hydroxy-4-pentadecylbenzaldehyde Moiety”. Adva. Chem., 2014; 1-9
- B.R. Thorat, P. Kamat, D. Khandekar, S. Lele, M. Mustapha, S. Sawant, R. Jadhav, S. Kolekar, R. Yamgar, R.G. Atram, “Synthesis and Fluorescence study of Novel Schiff Bases of Isoniazide”. J. Chem. Pharm. Res., 2011; 3(6): 1109-1117.
- Md. Saddam Hossain, C.M. Zakaria, Roushown Ali, Md. Kudrat-E-Zahan "Selected Pharmacological Applications of 1st Row Transition Metal Complexes: A review" Clin. Med. Res., 2017; 6(6): 177-191
- W.L. Liu, Y. Zou, C.L. Ni, Y.Z. Li, Q.J. Meng, “Synthesis, structural characterization and magnetic properties of new tripeptide Schiff base heterotrinuclear Cu(II)-M(II)-Cu(II) (M=Ni and Mn) complexes”. J. Molec. Struc., 2005; 751: 1–6
- P. Przybylski, G. Bejcar, G. Schroeder, B. Brzezinski, “Complexes of Schiff base of gossypol with 5-hydroxy-3-oxapentylamine and some monovalent cations studied by ESI MS as well as PM5 semiempirical methods,” J. Molec. Struc., 2003; 654(1-2): 245-252
- R. Dreos, G. Nardin, L. Randaccio, P. Siega, G. Tauzher, V. Vrdoljak, “Synthesis, molecular structure, and characterization in solution of a new series of inorganic and organometallic Co(III) Schiff base complexes”. Inorganica. Chimica. Acta, 2003; 349: 239–248
- R.K.Mohapatra, P.K.Das, M.K.Padhan, M.El-Ajaly, D.Das, H.F.Salem, U.Mahanta, G.Badhei, P.K.Parhi, A.A.Maihub and Md.Kudrat-E-Zahan "Recent Advances in Urea- and Thiourea-Based Metal Complexes: Biological, Sensor, Optical, and Corroson Inhibition Studies" Comm. Inorg. Chem., 2019; 0:1-61
- Md.Saddam Hossain, Md.Abdul Mannan, Md.Kudrat-E-Zahan "International Journal of Chemistry Studies Recent advances on microbial activity of metal complexes: A short review" Int. J. Chem. Stud., 2019; 4(1):17-24

