UTILIZING CARBON NANOTUBES AS EFFICIENT NANOADSORBENT FOR PANTOPRAZOLE REMOVAL FROM AQUEOUS SAMPLES: KINETICS, ISOTHERM, AND THERMODYNAMIC STUDIES

- Nanoadsorbent,
- Pantoprazole,
- Carbon nanotubes,
- Isotherm,
- Kinetics
- Thermodynamic ...More
Copyright (c) 2021 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
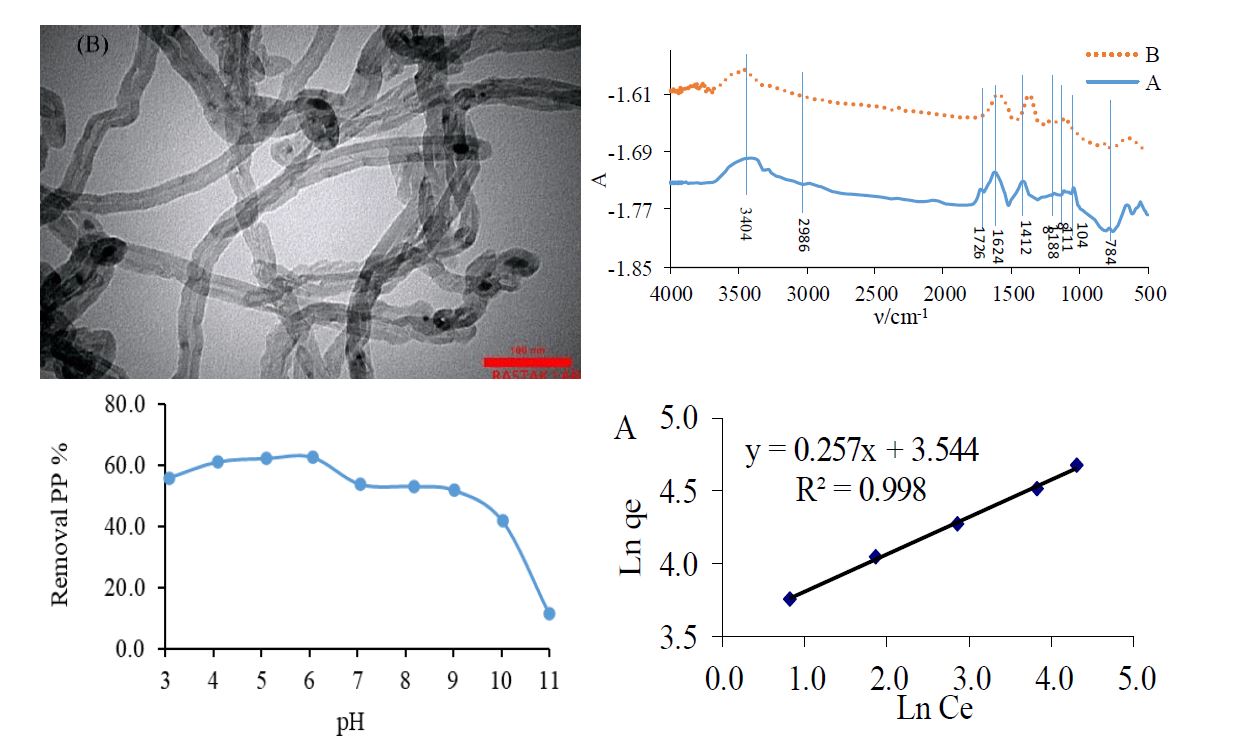
In this work, carbon nanotubes (MWCNTs) were utilized as efficient adsorbents for pantoprazole (PP) removal. We used MWCNTs that were synthesized using the chemical vapor deposition process. The physical characteristics of MWCNTs were described by Brunauer-Emmett-Teller (BET) contact area, surface functional group analysis by the point of zero charge (pHPZC), Fourier transform infrared (FTIR) analysis, Scanning electron microscope (SEM), X-ray diffraction (XRD), and Transmission electron microscopy (TEM). The single-point BET surface area of the MWCNTs was found to be 98.7 m2 g-1, with the median pores' diameter of 30.9 nm and an average pore(s) volume of 0.764 cm3 g-1. Effective parameters on the PP removal including, pH, contact time and initial amount of adsorbents were optimized, revealing maximum PP removal at pH=6.0 after 25.0 min when 0.026 g MWCNTs. The pseudo second-order kinetic model for adsorption of PP on the surface of both adsorbents revealed the high value of correlation coefficient, indicating the high ability of the pseudo second-order model for representation of experimental results. Adsorption equilibrium studies indicated that the Freundlich isotherm efficiently represented MWCNTs adsorption data. The thermodynamic parameters (Gebbs free energy, enthalpy, and entropy) of adsorption process were calculated. Results had shown that adsorption of PP on the MWCNTs is feasible, spontaneous, and exothermic process in the temperature range of 25-76 °C.
References
- Ali I, Al-Othman ZA, Alharbi OML. Uptake of pantoprazole drug residue from water using novel synthesized composite iron nano adsorbent. J Mol Liq. Elsevier; 2016;218:465–72.
- Zare MA, Husain SW, Tehrani MS, Azar PA. Pentaazatetraethylene supported polyacrylamide (PAA-N5) as a novel adsorbent for the efficient removal of industrial dyes from aqueous solutions: adsorption isotherms and kinetics. Monatshefte für Chemie-Chemical Mon. Springer; 2017;148:191–7.
- Khudhair AR, Sherazi STH, Al-Baiati MN. Adsorption of methylene blue from aqueous solutions by using a novel nano co-polymer. AIP Conf Proc. AIP Publishing LLC; 2020. p. 30021.
- Sarkar KK, Majee S, Pathak U, Mandal DD, Mandal T. Design and development of an integrated treatment system for pharmaceutical waste with toxicological study. Desalin Water Treat. 2019;5:75–85.
- Cavalcanti EB, Garcia-Segura S, Centellas F, Brillas E. Electrochemical incineration of omeprazole in neutral aqueous medium using a platinum or boron-doped diamond anode: degradation kinetics and oxidation products. water Res. Elsevier; 2013;47:1803–15.
- Sengupta A, Gupta NK. MWCNTs based sorbents for nuclear waste management: a review. J Environ Chem Eng. Elsevier; 2017;5:5099–114.
- Ali I, Aboul‐Enein HY, Kummerer K. Analyses of drugs and pharmaceuticals in the environment. Biophys Process Anthropog Org Compd Environ Syst. Wiley Online Library; 2011;439–62.
- Heberer T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicol Lett. Elsevier; 2002;131:5–17.
- Zhang Y, Geißen S-U, Gal C. Carbamazepine and diclofenac: removal in wastewater treatment plants and occurrence in water bodies. Chemosphere. Elsevier; 2008;73:1151–61.
- de García SO, Pinto GP, Encina PG, Mata RI. Consumption and occurrence of pharmaceutical and personal care products in the aquatic environment in Spain. Sci Total Environ. Elsevier; 2013;444:451–65.
- Kosma CI, Lambropoulou DA, Albanis TA. Analysis, occurrence, fate and risks of proton pump inhibitors, their metabolites and transformation products in aquatic environment: A review. Sci Total Environ. Elsevier; 2016;569:732–50.
- Barreiro JC, Vanzolini KL, Cass QB. Direct injection of native aqueous matrices by achiral–chiral chromatography ion trap mass spectrometry for simultaneous quantification of pantoprazole and lansoprazole enantiomers fractions. J Chromatogr A. Elsevier; 2011;1218:2865–70.
- Gracia-Lor E, Sancho J V, Serrano R, Hernández F. Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia. Chemosphere. Elsevier; 2012;87:453–62.
- Roy N, Alex SA, Chandrasekaran N, Mukherjee A, Kannabiran K. A comprehensive update on antibiotics as an emerging water pollutant and their removal using nano-structured photocatalysts. J Environ Chem Eng. Elsevier; 2020;104796.
- Leeladevi K, Kumar JV, Arunpandian M, Thiruppathi M, Nagarajan ER. Investigation on photocatalytic degradation of hazardous chloramphenicol drug and amaranth dye by SmVO4 decorated g-C3N4 nanocomposites. Mater Sci Semicond Process. Elsevier; 2020;105563.
- Ali A, Shoeb M, Li Y, Li B, Khan MA. Enhanced photocatalytic degradation of antibiotic drug and dye pollutants by graphene-ordered mesoporous silica (SBA 15)/TiO2 nanocomposite under visible-light irradiation. J Mol Liq. Elsevier; 2020;114696.
- Nakada N, Shinohara H, Murata A, Kiri K, Managaki S, Sato N, et al. Removal of selected pharmaceuticals and personal care products (PPCPs) and endocrine-disrupting chemicals (EDCs) during sand filtration and ozonation at a municipal sewage treatment plant. Water Res. Elsevier; 2007;41:4373–82.
- Pompei CME, Campos LC, da Silva BF, Fogo JC, Vieira EM. Occurrence of PPCPs in a Brazilian water reservoir and their removal efficiency by ecological filtration. Chemosphere. Elsevier; 2019;226:210–9.
- Wang L, Albasi C, Faucet-Marquis V, Pfohl-Leszkowicz A, Dorandeu C, Marion B, et al. Cyclophosphamide removal from water by nanofiltration and reverse osmosis membrane. Water Res. Elsevier; 2009;43:4115–22.
- Dolar D, Pelko S, Košutić K, Horvat AJM. Removal of anthelmintic drugs and their photodegradation products from water with RO/NF membranes. Process Saf Environ Prot. Elsevier; 2012;90:147–52.
- Oberoi AS, Jia Y, Zhang H, Khanal SK, Lu H. Insights into the fate and removal of antibiotics in engineered biological treatment systems: a critical review. Environ Sci Technol. ACS Publications; 2019;53:7234–64.
- Kanaujiya DK, Paul T, Sinharoy A, Pakshirajan K. Biological treatment processes for the removal of organic micropollutants from wastewater: a review. Curr Pollut Reports. Springer; 2019;5:112–28.
- Quesada HB, Baptista ATA, Cusioli LF, Seibert D, de Oliveira Bezerra C, Bergamasco R. Surface water pollution by pharmaceuticals and an alternative of removal by low-cost adsorbents: A review. Chemosphere. Elsevier; 2019;222:766–80.
- Mlunguza NY, Ncube S, Mahlambi PN, Chimuka L, Madikizela LM. Adsorbents and removal strategies of non-steroidal anti-inflammatory drugs from contaminated water bodies. J Environ Chem Eng. Elsevier; 2019;7:103142.
- Ali I, Gupta VK. Advances in water treatment by adsorption technology. Nat Protoc. Nature Publishing Group; 2006;1:2661.
- Ali I. Water treatment by adsorption columns: evaluation at ground level. Sep Purif Rev. Taylor & Francis; 2014;43:175–205.
- Dehmolaei A, Vadi M. Comparative Study of Adsorption Isotherms of Vitamin C on Multi Wall and Single Wall Carbon Nanotube. Orient J Chem. 2014;30:233–6.
- Kyzas GZ, Deliyanni EA. Modified activated carbons from potato peels as green environmental-friendly adsorbents for the treatment of pharmaceutical effluents. Chem Eng Res Des. Elsevier; 2015;97:135–44.
- Rasheed T, Bilal M, Hassan AA, Nabeel F, Bharagava RN, Ferreira LFR, et al. Environmental threatening concern and efficient removal of pharmaceutically active compounds using metal-organic frameworks as adsorbents. Environ Res. Elsevier; 2020;109436.
- Karimi-Maleh H, Shafieizadeh M, Taher MA, Opoku F, Kiarii EM, Govender PP, et al. The role of magnetite/graphene oxide nano-composite as a high-efficiency adsorbent for removal of phenazopyridine residues from water samples, an experimental/theoretical investigation. J Mol Liq. Elsevier; 2020;298:112040.
- Asfaram A, Ghaedi M, Azqhandi MHA, Goudarzi A, Dastkhoon M. Statistical experimental design, least squares-support vector machine (LS-SVM) and artificial neural network (ANN) methods for modeling the facilitated adsorption of methylene blue dye. RSC Adv. Royal Society of Chemistry; 2016;6:40502–16.
- Stobinski L, Lesiak B, Kövér L, Tóth J, Biniak S, Trykowski G, et al. Multiwall carbon nanotubes purification and oxidation by nitric acid studied by the FTIR and electron spectroscopy methods. J Alloys Compd. Elsevier; 2010;501:77–84.
- Dalvand A, Nabizadeh R, Ganjali MR, Khoobi M, Nazmara S, Mahvi AH. Modeling of Reactive Blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: Optimization, reusability, kinetic and equilibrium studies. J Magn Magn Mater. Elsevier; 2016;404:179–89.
- Kromer W. Similarities and differences in the properties of substituted benzimidazoles: a comparison between pantoprazole and related compounds. Digestion. Karger Publishers; 1995;56:443–54.
- El-Sherif ZA, Mohamed AO, El-Bardicy MG, El-Tarras MF. Reversed-phase high performance liquid chromatographic method for the determination of lansoprazole, omeprazole and pantoprazole sodium sesquihydrate in presence of their Acid-induced degradation products. Chem Pharm Bull. The Pharmaceutical Society of Japan; 2006;54:814–8.
- Senn-Bilfinger J, Krueger U, Sturm E, Figala V, Klemm K, Kohl B, et al. (H+-K+)-ATPase inhibiting 2-[(2-pyridylmethyl) sulfinyl] benzimidazoles. 2. The reaction cascade induced by treatment with acids. Formation of 5H-pyrido [1’, 2’: 4, 5][1, 2, 4] thiadiazino [2, 3-a] benzimidazol-13-ium salts and their reactions with thiols. J Org Chem. ACS Publications; 1987;52:4582–92.
- Danish M, Hashim R, Ibrahim MNM, Sulaiman O. Characterization of physically activated acacia mangium wood-based carbon for the removal of methyl orange dye. BioResources. 2013;8:4323–39.
- Wang P, Cao M, Wang C, Ao Y, Hou J, Qian J. Kinetics and thermodynamics of adsorption of methylene blue by a magnetic graphene-carbon nanotube composite. Appl Surf Sci. Elsevier; 2014;290:116–24.
- Chen Z, Fu J, Wang M, Wang X, Zhang J, Xu Q. Adsorption of cationic dye (methylene blue) from aqueous solution using poly (cyclotriphosphazene-co-4, 4′-sulfonyldiphenol) nanospheres. Appl Surf Sci. Elsevier; 2014;289:495–501.
- Aharoni C, Ungarish M. Kinetics of activated chemisorption. Part 2.—Theoretical models. J Chem Soc Faraday Trans 1 Phys Chem Condens Phases. Royal Society of Chemistry; 1977;73:456–64.
- Weber WJ, Morris JC. Kinetics of adsorption on carbon from solution. J Sanit Eng Div. ASCE; 1963;89:31–60.
- Lagergren S, Lagergren S, Lagergren SY, Sven K. Zurtheorie der sogenannten adsorption gelösterstoffe. 1898;
- Ho YS, McKay G. Pseudo-second order model for sorption processes. Process Biochem [Internet]. 1999;34:451–65. Available from: http://www.sciencedirect.com/science/article/pii/S0032959298001125
- Nakkeeran E, Saranya N, Giri Nandagopal MS, Santhiagu A, Selvaraju N. Hexavalent chromium removal from aqueous solutions by a novel powder prepared from Colocasia esculenta leaves. Int J Phytoremediation. Taylor & Francis; 2016;18:812–21.
- Freundlich HMF. Over the adsorption in solution. J Phys Chem. 1906;57:1100–7.
- Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc. ACS Publications; 1918;40:1361–403.
- Freundlich H. Über die Adsorption in Lösungen. Habilitationsschrift durch welche... zu haltenden Probevorlesung" Kapillarchemie und Physiologie" einladet Dr. Herbert Freundlich. W. Engelmann; 1906.
- Khayyun TS, Mseer AH. Comparison of the experimental results with the Langmuir and Freundlich models for copper removal on limestone adsorbent. Appl Water Sci. Springer; 2019;9:170.
- Kumar PS, Ramalingam S, Sathyaselvabala V, Kirupha SD, Murugesan A, Sivanesan S. Removal of cadmium (II) from aqueous solution by agricultural waste cashew nut shell. Korean J Chem Eng. Springer; 2012;29:756–68.
- Fytianos K, Voudrias E, Kokkalis E. Sorption–desorption behaviour of 2, 4-dichlorophenol by marine sediments. Chemosphere. Elsevier; 2000;40:3–6.
- Langmuir I. The constitution and fundamental properties of solids and liquids. II. Liquids. J Am Chem Soc. ACS Publications; 1917;39:1848–906.
- Foo KY, Hameed BH. Insights into the modeling of adsorption isotherm systems. Chem Eng J. Elsevier; 2010;156:2–10.
- Gehlot G, Verma S, Sharma S, Mehta N. Adsorption isotherm studies in the removal of malachite green dye from aqueous solution by using coal fly ash. Int J Chem Stud. 2015;3:42–4.
- Tempkin MI, Pyzhev V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys Chim USSR. 1940;12:327.
- Akkaya G, Özer A. Biosorption of Acid Red 274 (AR 274) on Dicranella varia: Determination of equilibrium and kinetic model parameters. Process Biochem. 2005;40:3559–68.
- Ayawei N, Ebelegi AN, Wankasi D. Modelling and Interpretation of Adsorption Isotherms. Guo W, editor. J Chem [Internet]. Hindawi; 2017;2017:3039817. Available from: https://doi.org/10.1155/2017/3039817
- Dubinin Mm. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem Rev. ACS Publications; 1960;60:235–41.
- Hobson JP. Physical adsorption isotherms extending from ultrahigh vacuum to vapor pressure. J Phys Chem. ACS Publications; 1969;73:2720–7.
- Chopra I, Singh SB. Kinetics and equilibrium study for adsorptive removal of cationic dye using agricultural waste-raw and modified cob husk. Int J Environ Anal Chem. Taylor & Francis; 2020;1–22.
- Senturk HB, Ozdes D, Gundogdu A, Duran C, Soylak M. Removal of phenol from aqueous solutions by adsorption onto organomodified Tirebolu bentonite: Equilibrium, kinetic and thermodynamic study. J Hazard Mater. Elsevier; 2009;172:353–62.