Copyright (c) 2017 Víctor Macías-Villamizar, Luís Cuca-Suárez

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
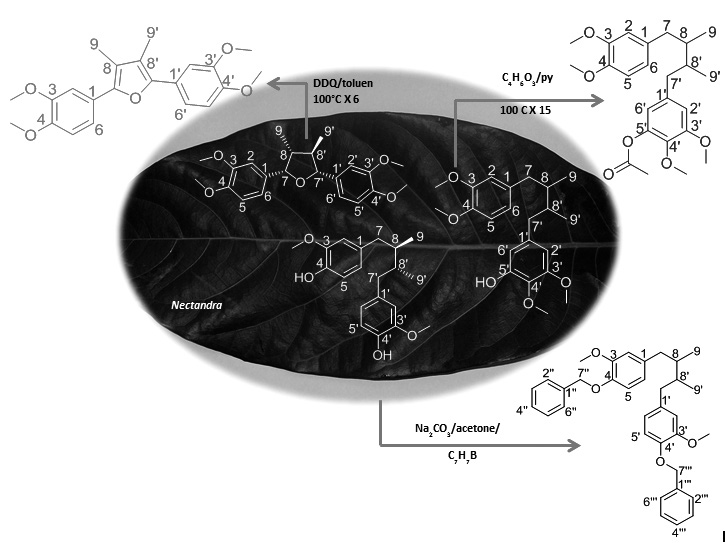
In the genus Nectandra, the presence of certain types of secondary metabolites has been determined, including sesquiterpenes, phytosterols, polyalcohols, arylpropionic acid derivatives, flavonols, arylpropanoids, furofuran lignans, dihydrobenzofuran neolignans [1], and certain norlignans [2], alkaloids [3], tannins [4], diterpenes [5], and components of essential oils [6]. However, the chemotaxonomic characteristics are determined by the presence of lignan-type compounds [7]. The ultimate goal of structural modification of natural products is to obtain new drugs [8]. In that sense, there is a growing interest in lignans and their synthetic derivatives due to applications in cancer chemotherapy and various other pharmacological effects [9]. This work corresponds to the first report of this type of structural modification of lignan compounds (7,7’-epoxylignans and diaryldimethylbutane lignans) isolated from Nectandra species. Therefore, this work can be used as a starting point for structure-activity relationship studies.
References
- J. M. Barbosa-Filho, M. Yoshida, O. R. Gottlieb, Phytochemistry. 28, 1991, (1989).
- L. Chérigo, V. Polanco, E. Ortega-Barria, M. V. Heller, T. L. Capson, L. C. Rios, Nat. Prod. Res. 19, 373, (2005).
- A. A. da Silva Filho, S. Albuquerque, M. L. e. Silva, M. N. Eberlin, D. M. Tomazela, J. K. Bastos, J. Nat. Prod., 67, 42, (2004).
- S. R. Farias-Moreno, A. Arnobio, J. José de Carvalho, A. L. Nascimento, M. O. Timoteo, B. Olej, E. K. Rocha, M. Pereira, M. Bernardo-Filho, L. Querino de Araújo Caldas, Biol. Res. 40, 131, (2007).
- J. C. Moro, J. B. Fernandes, P. C. Vieira, M. Yoshida, O. R. Gottlieb, H. E. Gottlieb, Phytochemistry, 26, 269, (1987).
- B. Agius, M. Setzer, S. Stokes, T. Walker, W. Haber, W. Setzer, Int. J. Essen. Oil Ther. 1, 167, (2007).
- J. G. Rohwer. Lauraceae: Nectandra. Flora Neotropica, Monograph 60, in Flora Neotropica Monograph. vol. 60, T. N. Y. B. Garden, Ed., ed New York, pp. 1-332, 1993.
- J. Chen, W. Li, H. Yao, J. Xu, Fitoterapia. 103, 231, (2015).
- M. Saleem, H. J. Kim, M. S. Ali, Y. S. Lee, Nat. Prod. Rep, 22, 696, (2005).
- L. Dalla Via, E. Uriarte, E. Quezada, A. Dolmella, M. G. Ferlin, O. Gia, J. Med. Chem. 46, 3800, (2003).
- R. Nakamura, Y. Obora, Y. Ishii, Tetrahedron. 65, 3577, (2009).
- H. S. P. Rao, S. Senthilkumar, J. Chem. Sci. 113, 191, (2001).
- L. McMaster, W. Bruner, Ind. Eng. Chem. 28, 505, (1936).
- M. Miyazawa, H. Kasahara, H. Kameoka, Phytochemistry. 46, 1173, (1997).
- Y. B. Xue, Y. L. Zhang, J. H. Yang, X. Du, J. X. Pu, W. Zhao, X. N. Li, W. L. Xiao, H. D. Sun, Chem. Pharm. Bull. 58, 1606, (2010).


