EFFECT OF GREEN SYNTHESIZED SILVER NANOPARTICLES ON CARNOSIC ACID CONTENT AND PHYSIO-BIOCHEMICAL PROPERTIES OF ROSMARINUS OFFICINALIS L.

- Keywords: Carnosic acid, Growth factors, Rosmarinus officinalis L, Silver nanoparticles.
Copyright (c) 2021 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
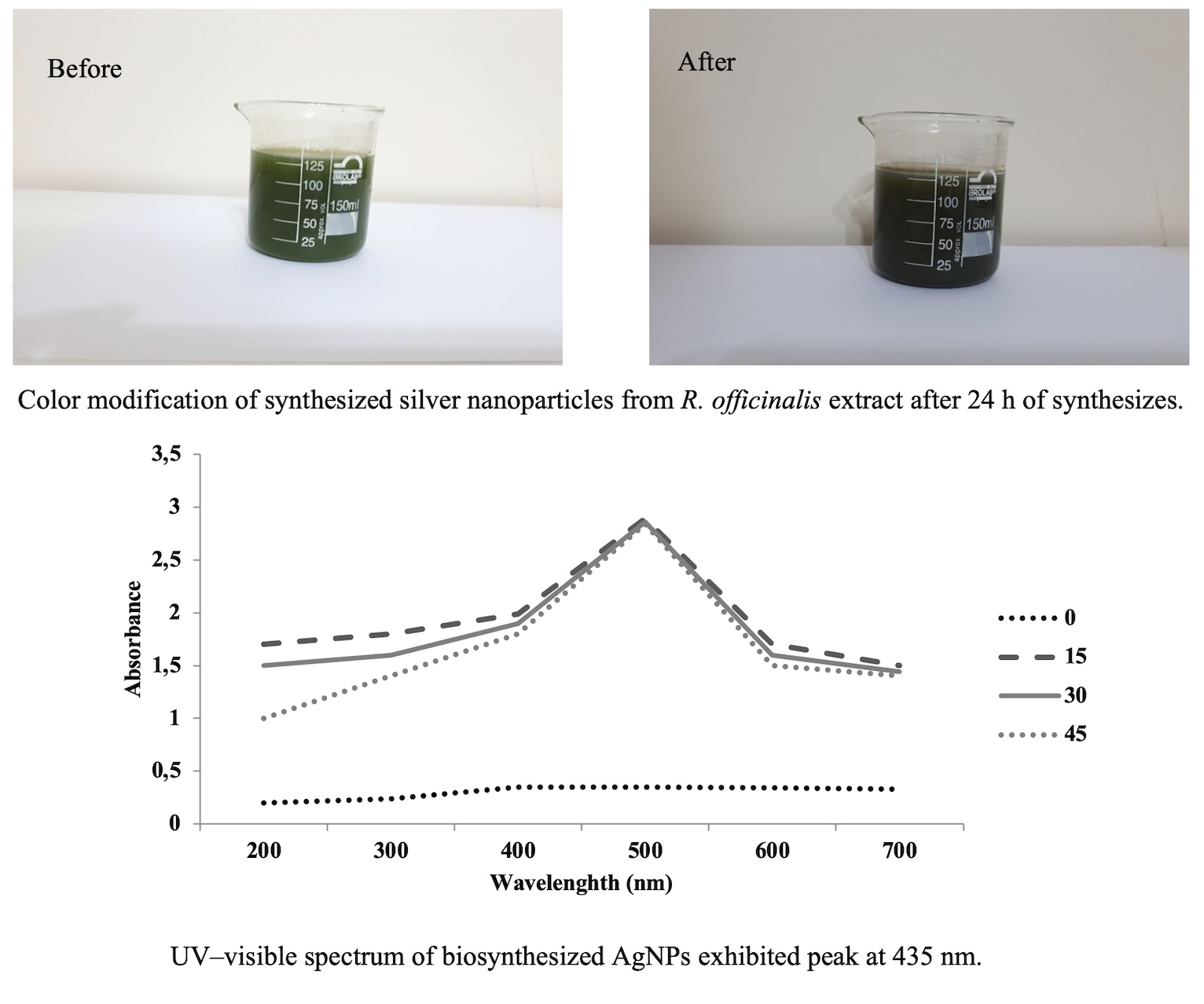
In biosynthesis of metal nanoparticles, it is possible to replace the old chemical synthesis methods with new environment-friendly procedures. In this study, AgNP was synthesized using extracts of Rosmarinus officinalis L. leaves, and then its effect on biochemical characters, antioxidant enzymes activity, and finally carnosic acid content was studied with its various concentrations. UV–visible spectroscopy was utilized to identify the formation of synthesized AgNP. Synthesized AgNP was confirmed the absorption maxima at 500 nm wavelength. Synthesized AgNP were measured using SEM and showed 21 nm at size and spherical shape. Application of various concentrations of synthesized AgNP demonstrated the concentration dependency so that, in concentration of 40 and 60 mM of synthesized AgNP, dry and fresh weight increased. Application of synthesized AgNP at 40 mM resulted in increase of chlorophyll amount which caused more activity in anti-oxidant enzymes and also biomass accumulation such as soluble carbohydrate and flavonoid. After treatment of synthesized AgNP at 40 mM concentration, HPLC demonstrated an increase in carnosic acid. Here, small nanoparticles revealed increased in secondary metabolites. Synthesized nanoparticles can also be utilized in order to produce natural products and plants with faster growth rates.
References
- References
- J.H. Chipault, G.R. Mizuno, J.M. Hawkins, W.O. Lundberg, J. Food Sci. 17 (1952).
- F. Aguilar, H. Autrup, S. Barlow, L. Castle, R. Crebelli, W. Dekrant, K.H. Engel, N. Gontard, D. Gott, S. Grilli, R. Gurtler, J.C. Larsen, C. Leclerq, J. Leblanc, F.X. Malcata, W. Mennes, M.R. Milana, I. P ratt, I. Rietjens, P. Tobback, F. Toldra, EFSA J 721 (2008).
- V. Calabrese, G. Scapagnini, C. Catalano, F. Dinotta, D. Geraci, P. Morganti, Int. J. Tissue React 22 (2000).
- C. Schluttenhofer, and L. Yuan, Plant Physiol 167 (2015).
- X. Li, J. Du, Y. Ou, H. Xu, X. Chen, A. Zhou, L. He, Y. Cao, Eur. Food Res. Technol 237 (2013).
- M.S. Bandara, K.K. Tanino, S.N. Acharya, (Editors) Advances in medicinal plant research, in: Acharya SN, Thomas JE (Eds), Rosemary (Rosmarinus officinalis L.): A Medicinal Plant Species. Research Signpost, India (2007).
- O.I. Aruoma, J.P. Spencer, R. Rossi, R. Aeschbach, A. Khan, N. Mahmood, A. Munoz, A. Murcia, J. Butler, B. Halliwell, Food Chem. Toxicol 34 (1996).
- S. Birtić, Carnosic acid. Phytochemistry 115) 2015).
- F.M. Amin, C.K. Marsha, M.E. Oussama, M.K. Sofian, Nano Sci. Nanotechnol 4 (2014).
- S. Iravani, H. Korbekandi, S.V. Mirmohammadi, B. Zolfaghari, Synthesis of silver nanoparticles: chemical, physical and biological methods Res Pharm Sci 9(6) (2014).
- A. Hebeish, M.E. El-Naggar, M.G. Moustafa, M.A. Ramadanb, S. Salem, M.H. El-Rafie, Highly effective antibacterial textiles containing green synthesized silver nanoparticles, Carbohydrate Polymers 136 (2) (2016).
- A. Kholood, A. Dahlous, H. Omar, Abd-Elkader, M.G. Moustaf, Fouda, A. Zeid. ALOthmana, Ayman El-Fahama, Journal of the Taiwan Institute of Chemical Engineers 95 (2019).
- A. Hebeishb, M.E. El-Naggar, M.G. Moustafa, M.A. Foudaa, Ramadan, S. Salem. M.H. Al-Deyabc, El-Rafie b, Carbohydrate Polymers 86 (2) (2011).
- . L.Y. Mehmet, E.N. Tanju, S.W. Atarb, Chemical Engineering Journal 242 (2014).
- M.L. Yola, Gupta, K. Vinod, T. Eren, A.E. Şen, N. Atar, Electrochimica Acta 120 (2014).
- A. Necip, E. Tanju, D. Bermali, L.Y. Mehmet, O. Ç. Mustafa, Ionics 21 (2015).
- A.S. K. John, S.T. Ziad, R. Job, N.A. F. Alnaizy, P. Howard, ENV Sci, Pollution Res. 22 (2015).
- E. Tanju, A. Necip, L.Y. Mehmet, K.M. Hassan, T.Ç. Alper, O. Asim, Ionics 21 (2015).
- M. Sofian, M.C. Kanan, H.H. Kanan, Res. Chem. Int 32 (2006).
- . N. Asare, C. Instanes, W. J. Sandberg, M. Refsnes, P. Schwarze, M. Kruszewski, and G. Brunborg, Toxicol 291 (2012).
- N. Okamura, Journal of Chromatography A 679(2) (1994).
- D.I Arnon, Plant Physiol 24 (1949).
- J.D. Hiscox, G.F. Can. J. Bot 57(1979).
- E.W. Yemm, A.J.Willis, Biochem 57 (1954).
- J. Zhishen, T. Mengcheng, W.u. Jianming, Food. Chem 64 (1999).
- H. Aebi, H. Bergmeyer, Verlag Chemie, Weinheim. Germany 3 (1983).
- B. Chance, A.C. Maehly, Methods biochem annals 1 (1954).
- J.S. Kim, E. Kuk, K.N. Yu, Nanomedicine 3 (2007).
- D.P. Briskin, Plant Physiol 124 (2000).
- A. Pancek, L. Kvtek, R. Prucek, M. Kolr, R. Vecerov, N. Pizorov, V.K. Sharma, T. Nevecn, R. Zboril, J. Phys. Chem. B 110 (33) (2006).
- Y. Zhou, W. Lin, J. Huang, W. Wang, Y. Gao, L. Lin, Q. Li, L. Lin, M. Du, Nanoscale Res Lett 5 (2010).
- P. Moteriya, H. Padalia, R. Jadeja, S. Chanda, Review: screening of silver nanoparticle synthetic efficacy of some medicinal plants of Saurashtra region. In: Gupta VK, editor. Natural products: research review, vol. 3.New Delhi: Daya Publishing House (2016).
- D. Lim, J.Y. Roh, H.J. Eom, J.Y. Choi, J. Hyun, and J. Choi, Environ. Toxicol. Chem 31 (2012).
- H. Qian, X. Peng, X. Han, J. Ren, Fu.Z. Sun, J. Environ. Sci 25 (2013).
- F. Perreault, M. Samadani, D. Dewez, Nanotoxicol 8 (2014).
- H.M.H. Salama, Int. Res. J. Biotechnol 3 (2012).
- P. Sharma, D. Sharma, M.G.H. Bhatt, P.P. Zaidi, P.K. Saradhi, S. Khanna, Appl. Biochem. Biotechnol 167 (2012).
- J.C. Tarafdar, and R. Raliya, Agri Res 2 (2013).
- A.O. Govorov, I. Carmeli, Nano Lett 7(3) (2007).
- M.H. Siddiqui, M.H. Al-Whaibi, M. Faisal, A.A. Al Sahli, Environ. Toxicol. Chem 33 (2014).
- M. Qi, Y. Liu, T. Li, Biological Trace Element Research 156 (2013).
- M. Kalteh, Z.T. Alipour, S. Ashraf, M.M. Aliabadi, A.F. Nosratabadi, J. Chem. Health Risks 4 (2014).
- D.K. Tiwari, N. Dasgupta-Schubert, L.M. Villaseñor-Cendejas, J. Villegas, L. Carreto Montoya, and S.E. Borjas-García, Applied Nanoscience 4 (2014).
- N. Rezvani, A. Sorooshzadeh, N. Farhadi, Int. J. Biol. Biomol. Agric. Food. Biotechnol. Eng 6 (2012).
- Y. Syu, J.H. Hung, J.C. Chen, Plant Physiol. Biochem 83 (2014).
- F. Arase, H. Arase, M. Nishitani, N. Egusa, S. Nishimoto, N. Sakurai, H. Sakamoto, I. Kaminaka, PLoS ONE 7 (2012).
- C. Vannini, G. Vannini, E. Domingo, B. Onelli, M. Prinsi, L. Marsoni, M. Espen, Bracale. PLoS ONE 8 (2013) e68752.
- C. Krishnaraj, E.G. Jagan, R. Ramachandran, S.M. Abirami, N. Mohan, P.T. Kalaichelvan, Plant growth metabolism, Process Biochemistry 47 (2012).
- J. Zhao, K. Sakai, Journal of Experimental Botany 54(383) (2003).
- Xu. Sh, T. Lou, N. Zhao, Y. Gao, L. Dong, D. Jiang, W. Shen, L. Huang, and R.Wang, Acta Physiol. Plant 33 (2011).
- M. Kumari, A. Mukherjee, N, Sci. Total Environ 407(2009).
- P.V. Asharani, Y.L. Wu, Z. Gong, S.Valiyaveettil, Nanotechnology 19) 2008).
- A. Kumar, V. Nirmala, Indian J Pharmaceut Sci 66(5) (2004).
- E. Navarro, A. Baun, R. Behra, Ecotoxicol 17 (2008).
- Y.S. El-Temsah, E.J. Joner, Toxicol 27(2012).
- N. Savithramma, S. Ankanna, G. Bhumi, Nano Vision 2 (2012).
- M. Ramezani, F. Ramezani, F. Rahmani, A. Dehestani, sci horti j 234 (2017).
- M. Ramezani, F. Rahmani, A. Dehestani, sci horti j 225 (2017).
- F. Daayf, M. Ongena, R. Boulanger, I.E. Hadrami, and R.R. Belanger, J. Chem. Ecol 26 (2000).
- S. Garcia-Sanchez, I. Bernales, and S. Cristobal, BMC Genomics 16 (2015).
- M. Comotto, A.A. Casazza, B. Aliakbarian, V. Caratto, M. Ferretti, and P. Perego, Sci. World J 9 (2014).
- M. Ghorbanpour, and J.Hadian, Carbon 94 (2015).
- K. Vecerova, Z. Vecera, B. Docekal, M. Oravec, A. Pompeiano, J. Tríska, Pollut 218 (2016).
- R. Suriyaprabha, G. Karunakaran, R. Yuvakkumar, V. Rajendran, N. Kannan, Curr Nanosci 8(6) (2005).
- B. Jasim, R. Thomas, J. Mathew, E.K. Radhakrishnan, Saudi Pharm. J 25 (2017).
- C. Krishnaraj, E.G. Jagan, R. Ramachandran, S.M. Abirami, N. Mohan, P.T. Kalaichelvan, Plant growth metabolism, Process Biochemistry 47 (2012).
- M. Kalteh, Z.T. Alipour, S. Ashraf, M.M. Aliabadi, A.F. Nosratabadi, Journal of Chemical Health Risks 4 (2014).
- E. Kohan-Baghkheirati, and J.Geisler-Lee, Nanomaterials 5 (2015).
- A. Sosan, D. Svistunenko, D. Straltsova, K. Tsiurkina, I. Smolich, T. Lawson, Plant J 85 (2016).
- M.N. Kha, M. Mobin, Z.K. Abbas, K.A. Almutairi, and Z.H. Siddiqui, Plant Physiol. Biochem 110 (2017).
- C. Schluttenhofer, and L. Yuan, Plant Physiol 167 (2015)
- U.J. Phukan, G.S. Jeena, and R.K. Shukla, Front. Plant Sci 7 (2016).
- Lim, D., Roh, J.Y, Eom, H.J., Choi, J.Y., Hyun, J., and Choi, J. Environ. Toxicol. Chem 31 (2012).
- Z. Majlesi, M. Ramezani, M. Gerami, Pharmaceutical and Biomedical Research 4(1) (2018).
- E.N. Frankel, Journal of Agricultural and Food Chemistry 44(1) (1996).

