ANTIMICROBIAL AND SOD-LIKE ACTIVITIES OF N,N′-BIS(FERROCENYLMETHYLENE)ETHYLENEDIAMINE SCHIFF BASE AND IT’S METAL COMPLEXES

- Antimicrobial activity,
- Ferrocene,
- Schiff base,
- Schiff base metal complex,
- SOD-like activity
Copyright (c) 2021 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
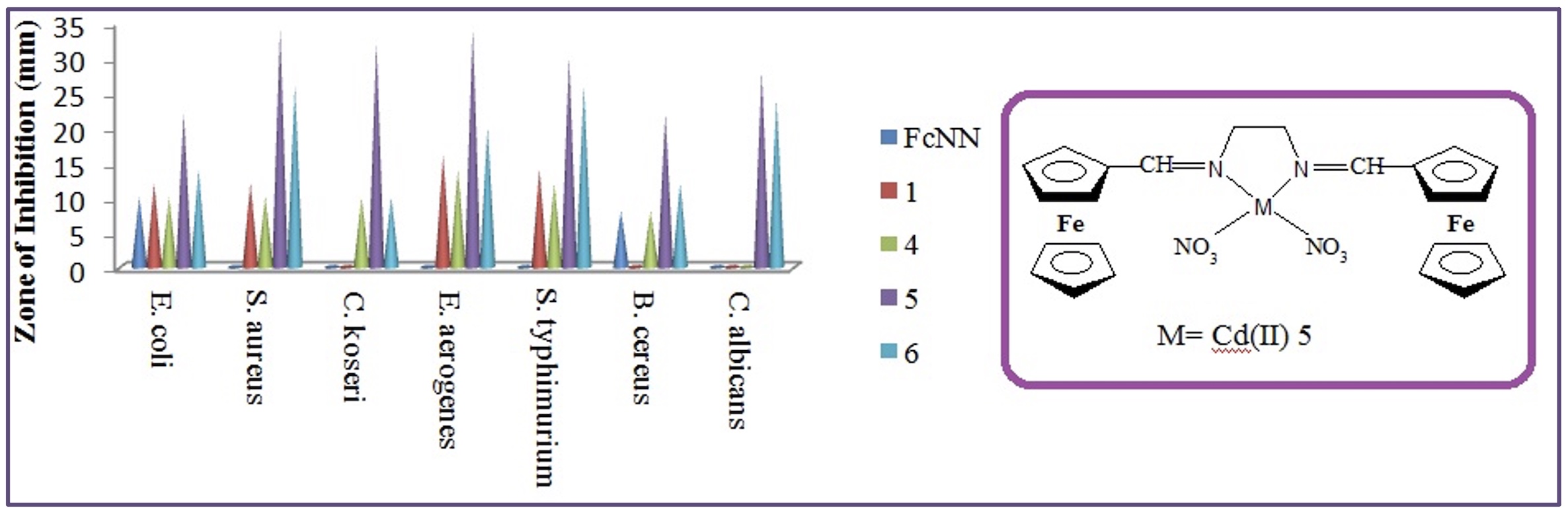
The synthesized N,N′-bis(ferrocenylmethylene)ethylenediamine Schiff base and it’s metal complexes were screened in vitro for antimicrobial activity against two Gram-positive (Staphylococcus aureus ATCC 25923, Bacillus cereus ATCC 7064),four Gram-negative (Escherichia coli ATCC 25922, Citrobacter koseri DBCC 01, Entorebacter aeruginosa ATCC 13048,Salmonella typhimurium DBCC 02) and antifungal activity against a Candida albicans. MIC values of the compounds ranged from 27 to 533 µg/mL. Compound (5) showed very strong activity against S. aureus with the best MIC (27 µg/mL). The lowest MIC for C. albicans was obtained 107 µg/mL. The superoxide dismutase activity of Schiff base and it’s metal complexes has been measured and discussed. SOD-like activities of bis ferrocenyl Schiff base ligand metal complexes were investigated using NBT. While only the Cu (II) complex showed SOD-like (40 μm IC50) activity, the SOD-like activity value was not determined in the other complex and ligand.
References
- E. Fluck, Gmelin Handbook of Inorganic and Organometallic Chemistry, eighth ed. Fe Organic Compounds, Berlin: Springer, 1991.
- A. Togni, and T. Hayashi, Ferrocenes, Homogenous Catalysis, Organic Synthesis, Material Science, Germany: VCH Publishers, Weinheim, 1995.
- P.D. Beer, J.E. Nation, M.E. Harman, M.B. Hursthouse, J. Organomet. Chem. 441, 465, (1992)
- A.J. Moore, P.J. Skabara, M.R. Bryce, A.S. Batsanov, J.A.K. Howard, S.T.A.K. Daley, J. Chem. Soc., Chem. Commun. 4, 417, (1993)
- P.D. Beer, A.R. Graydon, A.O.M. Johson, D.K. Smith, Inorg. Chem. 36, 2112, (1997)
- P.D. Beer, D.R.J. Smith, J. Chem. Soc., Dalton Trans. 417, (1998)
- (a) G.G.A. Balavoine, J-C. Daran, G. Iftime, P.G. Lacroix, E. Manoury, Organometallics, 18, 21, (1999) (b) I. Cuadrado, M.M. Carmen, M. Casado, B. Alonso, J. Losada, Coord. Chem. Rev. 193–195, 395, (1999)
- E.G. Morales-Espinoza, K.E. Sanchez-Montes, E. Klimova, T. Klimova, I.V. Lijanova, J.L. Maldonado, G. Ramos-Ortiz, S. Hernandez-Ortega, M. Martinez-Garcia, Molecules, 15, 2564 (2010)
- S.Celedon, M. Fuentealba, T. Roisnel, I. Ledoux-Rak, J-R. Hamon, D. Carrillo, C. Manzur, Eur. J. Inorg. Chem, 18, 3012, (2016)
- W. Yu, J. Jia, J. Gao, L. Han, Y. Li, Chem. Phys. Lett. 661, 251, (2016)
- H.J. Coles, S. Meyer, P. Lehmann, R. Deschenaux, I. Jauslin, J. Mater. Chem. 9, 1085, (1999)
- F.S. Koçak, C. Kavaklı, C. Akyol, M.A. Önal, S. Özkar, J. Organomet. Chem. 691, 5030, (2006)
- P. Deveci, B.Taner, Inorg. Chim. Acta. 405, 326, (2013)
- T. Akcan-Kardaş, H. Avcı-Özbek, Y. Akgül, F. Demirhan, Inorg. and Nano-Metal Chem. 47, 1475, (2017)
- M. Shabbir, Z. Akhter, I. Ahmad, S. Ahmed, M. Bolte, H. Ismail, B. Mirza, Inorg. Chim. Acta. 463, 102, (2017)
- J. Howarth, K. Hanlon, Bioorg. Med. Chem. Lett. 13, 2017 (2003)
- J. Howarth, K. Hanlon, Tetrahedron Lett. 42, 751 (2001)
- M.M. Abd-Elzaher, Appl. Organomet. Chem. 18, 149 (2004)
- A. Dallas, H. Kuhtz, A. Farrell, B. Quilty, K. Nolan, Tetrahedron Lett. 48, 1017, (2007)
- Y.T. Liu, G.D. Lian, D.W. Yin, B.J. Su, Spectrochim. Acta Part A: Molecular and Biomolecular Spectroscopy, 100, 131 (2013)
- Y. Li, X. Sun, D. Yin, Adv. Mater. Res. 339, 317 (2011)
- H. Avcı-Özbek, P. Sözen-Aktaş, J-C. Daran, M. Oskay, F. Demirhan, B. Cetinkaya, Inorg. Chim. Acta. 423, 435 (2014)
- Y-T. Liu, J. Sheng, D-W. Yin, H. Xin, X-M. Yang, Q-Y. Qiao, Z-J. Yang, J. Organomet. Chem. 856, 27 (2018)
- E.M. Njogu, B. Omondi, V.O. Nyamori, S. Afr. J. Chem. 69, 51, (2016)
- A.S. Hassan, T.S. Hafez, J. Appl. Pharm. Sci. 8 (05), 156 (2018)
- M.M. Abd-Elzaher, S.A. Moustafa, A.A. Labib, M.M. Ali, Monatsh Chem. 141, 387, (2010)
- M.M. Abd-Elzaher, S.A. Moustafa, A.A. Labib, H.A. Mousa, M.M. Ali, A.E. Mahmoud, Appl. Organomet. Chem. 26, 230 (2012)
- V.N. Babin, Y.A. Belousov, V.I. Borisov, V.V. Gumenyuk, Y.S. Nekrasov, L.A. Ostrovskaya, I.K. Sviridova, N.S. Sergeeva, A.A. Simenel, L.V. Snegura, Russ. Chem. Bull. (Int. Ed.), 63, 11, 2405 (2014)
- S. Gomez-Ruiz, D. Maksimovic-Ivanic, S. Mijatovic, G.N. Kaluderovic, Bioinorg. Chem. Appl. (2012)
- V. Janka, D. Zatko, V. Ladislav, P. Pal, P. Janka, M. Gabriela, In Vitro Cell. Dev. Biol.-Animal, 51, 964 (2015)
- E. Uddin, R. Islam, Ashrafuzzaman, N.A. Bitu, A. Asraf, F. Hossen, Md. K. E-Zahan, New Mater., Comp. Appl. 4, 63 (2020)
- A. Özel, K. Karaoğlu, K. Serbest, N. Gürcan, M. Emirik, U. Çoruh, J. Coord. Chem. 69, 1587, (2016)
- K. Kowalski, Coord. Chem. Rev. 317, 132, (2016)
- M. Emirik, K. Karaoğlu, K. Serbest, E. Menteşe, I. Yilmaz, J. Mol. Struct. 1106, 331, (2016)
- Z-Q. Liu, Mini-Rev. Med. Chem. 11, 345, (2011)
- C. Mu, K.E. Prosser, S. Harrypersad, G.A. MacNeil, R. Panchmatia, J.R. Thompson, S. Sinha, J.J. Warren, C.J. Walsby, Inorg. Chem. 57, 15247, (2018)
- R.A. Hussain, A. Badshahz, K. Akbar, Russ. J. Electrochem. 2015, 51, 198, (2015)
- E.W. Neuse, J. Inorg. Organomet. Polym. Mater. 15, 3, (2015)
- Y-F. Li, Z-Q. Liu, Eur. J. Pharm. Sci. 44, 158 (2011)
- D.P. Riley, Chem. Rev. 99, 2573, (1999)
- E.W. Neuse, M.G. Meirim, N.F. Blom, Organometallics, 7, 2562, (1988)
- P. Li, J.I. Scowen, E.J. Daives, A.M.J, Halcrow, Chem. Soc. Dalton Trans. 22, 3791, (1998)
- H. Zhang, J. Lei, Y. Chen, L. Lin, Q. Wu, Synth. React. Inorg. Met. Org. Chem. 31, 1053, (2001).
- H. Zhang, J. Lei, Y. Chen, Q. Wu, Y. Zhang, L. Gao, Synth. React. Inorg. Met. Org. Chem. 31, 937, (2001)
- H. Hou, G. Li, Y. Song, Y. Fan, Y. Zhu, L. Zhu, Eur. J. Inorg. Chem. 12, 2325, (2003)
- Z. Petrovski, S.S. Braga, S.S. Rodrigues, C.C.L. Pereia, I.S. Goncalves, M. Pillinger, C. Freire, C.C. Romao, New J. Chem. 29, 347, (2005)
- Z. Petrovski, S.S. Braga, A.M. Santos, S.S. Rodrigues, I.S. Goncalves, M. Pillinger, E.F. Kuhn, C.C. Romao, Inorg. Chim. Acta, 358, 981, (2005)
- W-J. Liu, G-X. Xiong, W-P.Wang, Appl. Organomet. Chem. 21, 83, (2007)
- J.M. Lloris, A. Benito, R. Martinez-Manez, M.E. Padilla-Tosta, T. Pardo, J. Soto, M.J.L. Tendero, Helvetica Chim. Acta, 81, 2024, (1998)
- A. Benito, J. Cano, R. Martinez-Manez, J. Soto, J. Paya, F. Lloret, M. Julve, J. Faus, M.D. Marcos, Inorg. Chem. 32, 1197, (1993)
- K.S. Pal, K. Alagesan, G.A. Samuelson, J. Pebler, J. Organomet. Chem. 575, pp. 108, (1999)
- D-J. Cho, S-J. Jeon, H-S. Kim, T-J. Kim, Synlett, 6, 617, (1998)
- W.H. Mahmoud, N.F. Mahmoud, G.G. Mohamed, Appl Organomet. Chem. 31, e3858, (2017)
- A.M. Elseman, A.E. Shalan, M.M. Rashad, A.M. Hassan, N.M. Ibrahim, A.M. Nassar, J. Phys. Org. Chem. 30, e3639, (2017)
- C. Perez, M. Pauli, P. Bazerque, Acta. Biol. Med. Exp. 15, 113, (1990)
- Clinical and Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically; approved standard M7-A6, 6thedn. National Committee for Clinical Laboratory Standards, Wayne, Pa. 2003.
- Clinical and Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A2. Second edition. National Committee for Clinical Laboratory Standards, Wayne, Pa. 2002.
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing, 16th informational supplement M100-S16. National Committee for Clinical Laboratory Standards, Wayne, Pa. 2006.
- C. Beauchamp, I. Fridovich, Anal. Biochem. 44, 276, (1971)
- R.N. Patel, K.K. Shukla, A. Singh, M. Choudhary, U.K. Chauhan, S. Dwivedi, Inorg. Chim. Acta. 362, 4891, (2009)
- Y.P. Singh, R.N. Patel, Y. Singh, R.J. Butcher, P.K. Vishakarma, R.K.B. Singh, Polyhedron, 122, 1, (2017)
- Y-H. Zhou, X-W. Liu, L-Q. Chen, S.-Q. Wang, Y. Cheng, Polyhedron, 117, 788, (2016)
- J. Joseph, G.A.B. Rani, Arabian J. Chem. 10, 1963, (2017)
- D. Klug, J. Rabani, I. Fridovich, J. Biol. Chem. 247, 4839, (1972)
- Z.A. Siddiqi, M. Shahid, M. Khalid, S. Kumar, Eur. J. Med. Chem. 44, 2517, (2009)
- A. Nebot-Guinot, A. Liberato, M.A. Máñez, M.P. Clares, A. Doménech, J. Pitarch-Jarque, A. Martínez-Camarena, M.G. Basallote, E. García-España, Inorg. Chim. Acta, 472, 139, (2018)


