POLY(4-VINYLBENZYL)TRIMETHYLAMMONIUM CHLORIDE) RESIN WITH REMOVAL PROPERTIES FOR VANADIUM(V) AND MOLYBDENUM(VI). A THERMODYNAMIC AND KINETIC STUDY
- Resins, removal, molybdenum, vanadium
Copyright (c) 2021 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
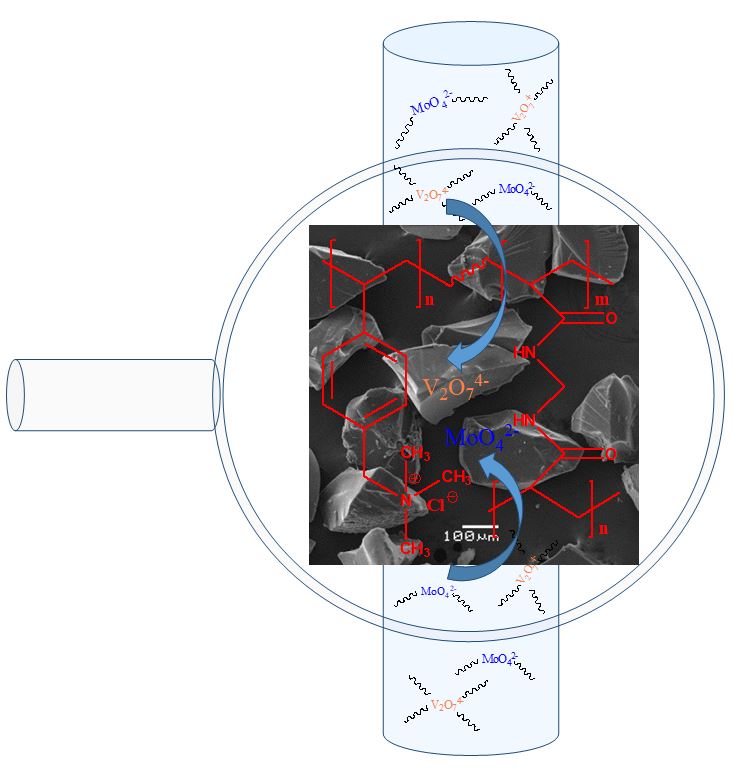
Ion exchange resin poly(4-vinylbenzyl) trimethylammonium chloride(P(ClVBTA)) was synthesized and its removal properties toward vanadium(V) and molybdenum(VI) were evaluated and compared with those of the Amberlite IRA-402 commercial resin. The resin was characterized by FT-IR spectroscopy, TGA, and SEM. The water absorption capacity, pH effect, Langmuir and Freundlich adsorption isotherms, and kinetic model parameters were determined. All studies were conducted through a batch equilibrium procedure. Thermodynamic parameters, including enthalpy, entropy, and free energy, were determined. The P(ClVBTA) resin showed faster and higher capacity for the removal of V(V) and Mo(VI) from a water solution than the Amberlite IRA-402 commercial resin with the same ammonium salt functional group. The higher capacity of the P(ClVBTA) resin was attributed to the higher degree of swelling, the exfoliation in the monolayer, and the small particle size.
References
2. Borbely, G., Nagy, E. Removal of Zinc and Nickel Ions by Complexation Membrane Filtration Process from Industrial Wastewater. Desalination2009, 240 (1–3), 218–226.
3. Korus, I. Loska, K. Removal of Cr(III) and Cr(VI) Ions from Aqueous Solutions by Means of Polyelectrolyte-Enhanced Ultrafiltration. Desalination2009, 247, 390–395.
4. Pedersen, A. J. Characterization and Electrolytic Treatment of Wood Combustion Fly Ash for the Removal of Cadmium. Biomass Bioenergy2003, 25 (4), 447–458.
5. Rivas, B. L.; Espinosa, C.; Sánchez, J. Application of the Liquid-Phase Polymer-Based Retention Technique to the Sorption of Molybdenum(VI) and Vanadium (V). Polym. Bull.2019, 76, 539–552.
6. Morales, D. V.; Kusku, O.; Rivas, B. L.; Muserref, A.; Kabay, N.; Bryjak, M. Removal of Cr(VI) by Stabilized Solvent Impregnated Resin(SIR) Prepared by Using a Hydrophilic Polymer Adsorbent and Aliquat 336. J. Chil. Chem. Soc.2019, 64, 4432–4436.
7. Núñez, D.; Serrano, J. A.; Mancisidor, A.; Elgueta, Elizabeth Kokkarachedu, V.; Oyarzún, P.; Cáceres, R.; Ide, W.; Rivas., B. L. Heavy Metal Removal from Aqueous Systems Using Hydroxyapatite Nanocrystals Derived from Clam Shells. RSC Adv.2019, 9, 22883–22890.
8. Sánchez, J.; Rivas., B. L. Liquid-Phase Polymer-Based Retention to Remove Arsenic from Water: A Mini Review. J. Chil. Chem. Soc.2019, 64, 4432–4522.
9. Tapiero, Y.; Sánchez, J.; Rivas, B. L. Activated Polypropylene Membranes with Ion-Exchange Polymers to Transport Chromium Ions in Water. J. Chil. Chem. Soc.2019, 64, 4597–4606.
10. Mitchell, P.C.H. Ullman’s Encyclopedia of Industrial Chemistry, 5th Ed.; VCH, Ed.; 1986.
11. Rivas BL, Pooley SA, Pereira ED, Cid R, Luna M, Jara, MA, Geckeler, KE
Water-soluble amine and imine polymers with the ability to bind metal ions in
conjunction with membrane filtration , J. Appl. Polym. Sci. 2005, 96, 222-231.
12. Sastre, A.M.; Alguacil, F. J. Co-Extraction and Selective Stripping of Copper(II) and Molybdenum(VI) Using LIX 622. Chem. Eng. J.2001, 81, 109 – 112.
13. Liansheng, X.; Qixiu, Z.; Bofan, G.; Shaoying, H. Separation of Molybdenum from Tungstate Solution by a Combination of Moving Packed Bed and Fluid Ion-Exchange Techniques. Int. J. Refract. Met. Hard Mater.2001, 19, 145 – 148.
14. Juneja, J.M.; Singh, S.; Bose, D. K. Investigations on the Extraction of Molybdenum and Rhenium Values from Low Grade Molybdenite Concentrate. Hydrometallurgy1996, 41, 201– 209.
15. Guibal, E.; Milot, C.; Roussy, J. Influence of Hydrolysis Mechanisms on Molybdate Sorption Isotherms Using Chitosan. Sep. Sci. Technol.2000, 35, 1021–1038.
16. Hua, T.; Haynesa, R. J.; Zhou, Y.-F.; Boullemant, A.; Chandrawana, I. Potential for Use of Industrial Waste Materials as Filter Media for Removal of Al, Mo, As, V and Ga from Alkaline Drainage in Constructed Wet Lands – Adsorption Studies. Water Res.2015, 71, 32–41.
17. Rivas B. L, Maturana H.A, Pereira, ED. Metal ion binding properties of synthetic
vinyl resins. Angew. Makromol. Chem. 1994, 220, 61-74.
18. Mitchell, P. C. H. Ullmann’s Encyclopedia of Industrial Chemistry; 1990; pp 675–682.
19. Aveston, J., Anacker, E.W. Johnson, J. S. Hydrolysis of Molybdenum (VI). Ultracentrifugation, Acidity Measurements, and Raman Spectra of Polymolybdates. Inorg. Chem.1964, 3 (5), 735–746.
20. Busey, R.H., and Keller, O. L. Structure of the Aqueous Pertechnetate Ion by Raman and Infrared Spectroscopy. Raman and Infrared Spectra of Crystalline KTcO4, KReO4, Na2MoO4, Na2WO4, Na2MoO4•2H2O, and Na2WO4•2H2O. J. Chem. Phys.1964, 41 (1), 215–225.
21. Bartecki, A. and Dembicka, D. Spectroscopy of Molybdenum(VI) Compounds. J. Inorg. Nucl. Chem. 1967, 29 (12), 2907–2916.
22. Roschin AV Toxicology of vanadium compounds used in modern industry.
Water Research 1967, 32, 26-32
23. Cotton, F.A. and Wilkinson, G. Advanced Inorganic Chemistry, 5th editio.; Sons, J. W. &, Ed.; 1988.
24. Kusku, O.; Rivas, B. L.; Urbano, B. F.; Arda, M.; Kabay, N.; Bryjak, M. A Comparative Study of Removal of Cr(VI) by Ion Exchange Resins Bearing Quaternary Ammonium Groups. J. Chem. Technol. Biotechnol.2014, 89 (6), 851–857.
25. Pérez, J.; Toledo, L.; Campos, C. H.; Yañez, J.; Rivas, B. L.; Urbano, B. F. Arsenic Sorption onto an Aluminum Oxyhydroxide-Poly[(4-Vinylbenzyl)Trimethylammonium Chloride] Hybrid Sorbent. RSC Adv.2016, 6 (34), 28379–28387.
26. Moraga, B.; Toledo, L.; Jelínek, L.; Yañez, J.; Rivas, B. L.; Urbano, B. F. Copolymer–Hydrous Zirconium Oxide Hybrid Microspheres for Arsenic Sorption. Water Res. 2019, 116, 115044-115054.
27. Demircioğlu, M,; Kocacık, N.; Yiğit, E. and K. N. Removal of Metal Pollutants (Cd,
U) from Phosphoric Acid Solutions by Ion Exchange II. Mathematical Modeling.
Innov. Miner. Coal Process.1998, 781–785.
28. Morales, D. V.; Rivas, B. L.; González, M. Synthesis and Characterization of Poly([(2-Methacryloyloxy) Ethyl]) Trimethylamonnium Chloride) Resin with Removal Properties for Vanadium(V) and Molybdenum(VI). J. Chil. Chem. Soc. 2016, 61 (4), 3295–3303.