APPROACHES FOR CHEMICAL SYNTHESIS AND DIVERSE PHARMACOLOGICAL SIGNIFICANCE OF PYRAZOLONE DERIVATIVES: A REVIEW

- Pyrazolones, biological activities, antidiabetic, antimicrobial, anti-inflammatory, cardioprotective, antioxidant, anticancer, heterocyclic compounds.
Copyright (c) 2021 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
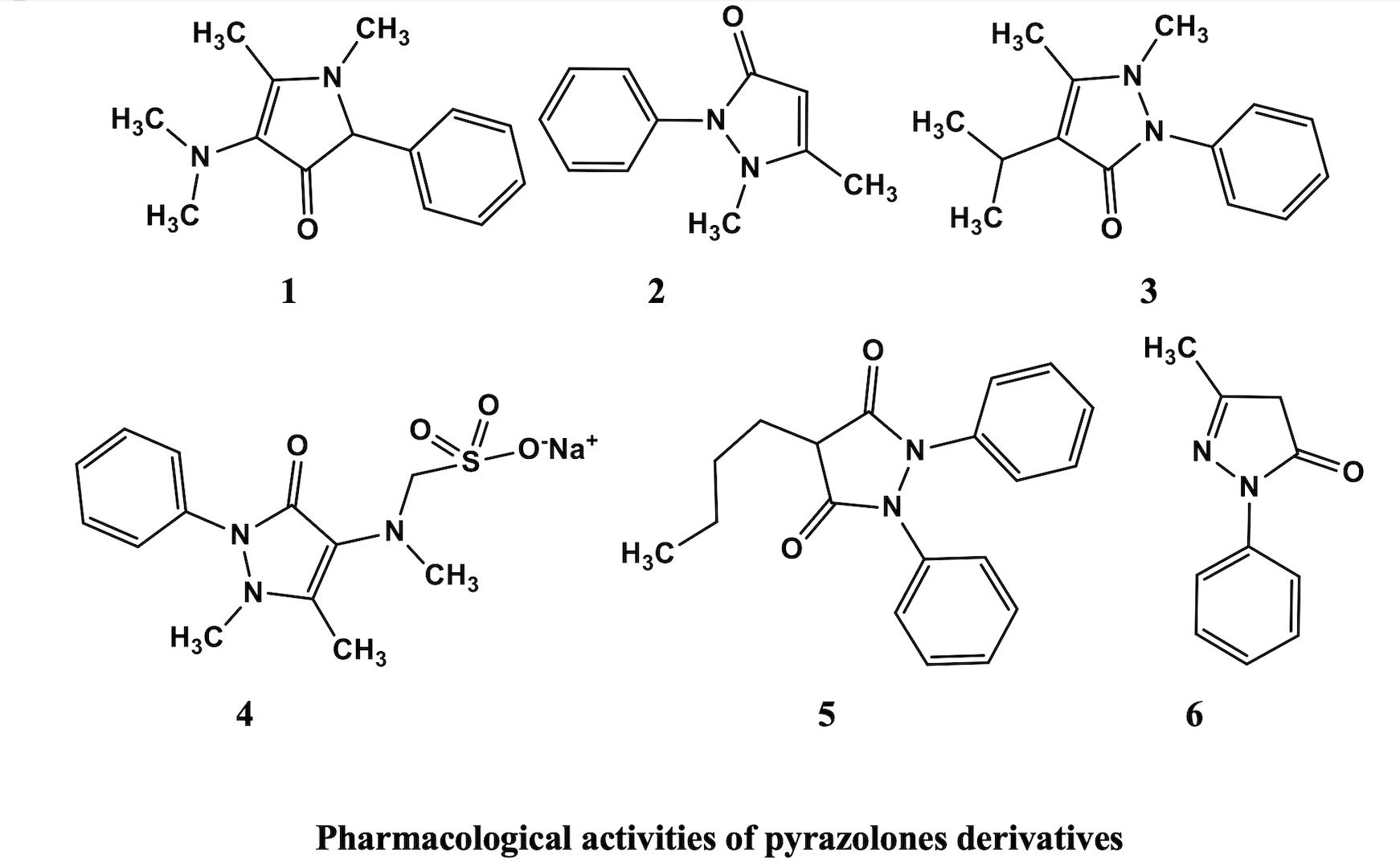
Pyrazolone is a five-membered lactam ring containing two Nitrogens and one ketonic group in its structure. Numerous pyrazolone derivatives were exhibited with diverse biological, pharmacological, and chemical applications. When pyrazolones were discovered, they were only known as NSAIDs but in recent times they play a versatile role in several complications like cerebral ischemia, cardiovascular diseases, antibacterial, antioxidant, anticancer and several other pharmacological activities. Over the last few decades, pyrazolone derivatives have been used for various biochemical applications. Some of these derivatives such as metamizole, phenazone, aminopyrine, and propyphenazone, are widely used as anti-inflammatory and analgesics. The chemistry of pyrazolone has gained increasing attention due to its diverse pharmacological properties such as anticancer, analgesic, anti-inflammatory, antimicrobial, antioxidant, antifungal, antiviral, antidiabetic, and several other biological activities. Thus, keeping because of their importance, synthetic strategies for existing as well as novel pyrazolone derivatives have been developed and explored their biochemical utility. This review deals with the various pharmacological properties of different pyrazolone derivatives and puts chemical synthetic schemes.
References
- Lednicer, D., and Mitscher, L.A. In Organic Chemistry of Drug Synthesis; Wiley Interscienc: New York, 1997, 1, 226.
- Lalit, K., Chandresh, T., Vivek, S. Inter. J. Res. Pharm. & Sci., 2012, 2(2), 13‒22.
- Yoon, S., Choi, B., Rahman, M.M., Kumar, S., Kabir, S.M.M., Koh, J. Materials, (Basel), 2019, 12(24), 4209.
- Idemudia, O.G., Sadimenko, A.P., Hosten, E.C. Inter. J. Mol. Sci., 2016, 17(5), 687.
- A‒mata, E., Bland, N. D., Campbell, R.K., Pollastri, M.P. Tetrahedron Lett., 2015, 56(21), 2832–2835.
- Idemudia, O.G., Sadimenko, A.P., Afolayan, A.J., Hosten, E.C. Bioinorg. Chem. Appl., 2015, 717089.
- Stefaniak, J., Lewis, A.M., Conole, D., Galan, S.R.G., Bataille, C.J.R., Wynne, G.M., Castaldi, M.P., Lundbäck, T., Russell, A.J., Huber, K.V.M. ACS Chem. Biol., 2018, 13(10), 2849–2854.
- Romina, N., Camilla, F., Serena, M., Elisabetta, C., Tiziano, T., Ilaria, M.M., Francesco, D.L., Delia, P., Raquel, T., Alberto, C., Riccardo, P., Pierangelo, G., Silvia, B. Br. J. Pharmacol., 2015, 172(13), 3397–3411.
- Paul, C.T., Kevin, T.Z., Susan, G.F., Isaac, T.S., Radhia, B., Jason. M., Donald, R.K., Richard, I.M., Richard, B.S. ACS Chem. Neurosci. 2014, 5(9), 823–829.
- Victor, H., Yung-Hyo, K., Tino, W.S., Danielle, B., Nouri, N., Kyung, W.J. Bioorg. Med. Chem. Lett., 2010, 20(22), 6854–6857.
- Yinan, Z., Radhia, B., He, H., Tian, C., Cindy, V., Richard, I.M., Donald, R.K., Richard, B. S. J. Med. Chem., 2013, 56(6), 2665–2675.
- Sumit S,M., Michele, S., Smitha, S., Jeff, P., Uyen, L., Taylor, K.L., Vid, L., Angel, T., Aaron, D. J. Med. Chem., 2014, 57(8), 3283–3294.
- Mei, Z., Zhi-fu, X., Run-tao, Z., Da-kai, C., Min, G., Shi-chao, C., Yang-ming, Z., Xin-wen, Z., Yan-yan, Y., Jia, L., Fa-jun, N., Jing-ya, L. Acta Pharmacol. Sin., 2018, 39(10), 1622–1632.
- Camila, M.-G., Daniela, C.-R., Francisco, A.-C., Iván, P., Eduardo, F., Julio, C. PLoS One, 2017, 12(12), e0189213.
- Ravindranath, L.K., Kumar, E.V.S., Srikanth, K., Spoorthi, Y.N., Phebe, P. Life Sci. Chem., 2012, 2(3), L-145.
- Himly, M., Jahn-Schmid, B., Pittertschatscher, K., Bohle, B., Grubmayr, K., Ferreira, F., Ebner, H., and Ebner, C. J. Allergy Clin. Immunol., 2003, 111, 882‒888.
- Al-Haiza, M.A., El-Assiery, S.A., Sayed, G.H. Acta Pharm., 2001, 51, 251‒261.
- Castagnolo, D., Manetti, F., Radi, M., Bechi, B., Pagano, M., De Logu, A., Meleddu, R., Saddi, M., and Botta, M. Bioorg. Med. Chem., 2009, 17(15), 5716‒5721.
- Radi, M., Bernardo, V., Bechi, B., and Castagnolo, D. Tetrahedron Lett., 2009, 50, 6572‒6575.
- Moreau, F., Desroy, N., Genevard, J.M., Vongsouthi, V., Gerusz, V., Le Fralliec, G., Oliveira, C., Floquet, S., Denis, A., Escaich, S., Wolf, K., Busemann, M., and Aschenbrenner, A. Bioorg. Med. Chem. Lett., 2008, 18(14), 4022‒4026.
- Sauzem, P.D., Machado, P., Rubin, M.A., da S Sant’anna, G., Faber, H.B., de Souza, A.H., Mello, C.F., Beck, P., Burrow, R.A., Bonacorso, H.G., Zanatta, N., and Martins, M.A. Eur. J. Med. Chem., 2008, 43(6), 1237‒1247.
- Pasha, F.A., Muddassar, M., Neaz, M.M., and Cho, S.J. J. Mol. Graph. Model., 2009, 28(1), 54‒61.
- Rosiere, C.E., and Grossman, M.I. Science, 1951, 113(2945), 651‒653.
- Bailey, D.M., Hansen, P.E., Hlavac, A.G., Baizman, E.R., Pearl, J., DeFelice, A.F., Feigenson, M.E. J. Med. Chem., 1985, 28(2), 256‒260.
- Chauhan, P.M., Singh, S., and Chatterjee, R.K. Indian J. Chem. Sect. B, 1993, 32, 858‒861.
- Shestopalov, A.M., Emelyanova, Y.M., Shestopalov, A.A., Rodinovskaya, L.A., Niazimbetova, Z.I., and Evans, D.H. Tetrahedron, 2003, 59, 491‒7496.
- Guangfei, L., Lang, Li., Dianzeng, J., and Kaibei, Y. Struct. Chem., 2005, 16, 135‒140.
- Fiordalisi, F.M. United State Patent Office. Patented Jan. 19, 1965, 3,166,475.
- Brune, M.D., Acute Pain, 1997, 1(1), 33‒40.
- Knorr, L. Ber Dtsch Chem. Ges., 1883, 16, 2597.
- Rainsford, K.D. J. Physiol., 2001, 95, 11‒19.
- Diptesh, S., Rishi, K., Ashoke, S., Prakas, R.M., Vishnu Ji R. Tetrahedron Lett., 2005, 46(22), 3807‒3809.
- Higashi, Y., Jitsuikia, D., Chayamab, K., Yoshizumia, M. Recent Patents on Cardiovas Drug Disc., 2006, 1, 85‒93.
- Mariappan. G., Saha, B.P., Bhuyan, N.R., Bharti, P.R., Deepak, K. J. Adv. Pharm. Tech. Res., 2010, 1(2), 260‒267.
- Baciu-Atudosie, L., Ghinet, A., Belei, D., Gautret, P., Rigo, B., Bicu, E. Tetrahedron Lett., 2012, 53, 6127‒6131.
- Ochei, J., Kolhatkar, A. Medicinal Laboratory Science-Theory and Pracitces; Tata McGrow-Hill Publishing Co. Ltd.: New Delhi, 2000, 808‒818.
- Isloor, A.M., Kalluraya, B., Sridhar Pai, K. Eur. J. Med. Chem., 2010, 45(2), 825‒830.
- Chandrakantha, B., Shetty, P., Nambiyar, V., Isloor, N., Isloor, A.M. Eur. J. Med. Chem., 2010, 45(3), 1206‒1210.
- Sankappa Rai, U., Isloor, A.M., shetty, P., Vijesh, A.M., Prabhu, N., Isloor, S., Thiageeswaran, M., Fun, H.K. Eur. J. Med. Chem., 2010, 45(6), 2695‒2699.
- Vijesh, A.M., Isloor, A.M., Isloor, S.K., Shivananda, K.N., Shyma, P.C., Arulmoli, T. Der. Pharm. Chem., 2011, 3, 454‒463.
- Yoshida, H., Yanai, H., Namiki, Y., Fukatsu-Sasaki; K., Furutani, N., Tada, N. CNS Drug Rev., 2006, 12(1), 09‒20.
- Yagi, H., Horinaka, S., Matsuoka, H. J. Cardiovasc. Pharmacol., 2005, 46(1), 46‒51.
- Hassan, A.E., Moustafa, A.H., Tolbah, M.M., Zohdy, H.F., Haikal, A.Z. Nucleosides Nucleotides Nucleic Acids, 2012, 31(11), 783‒800.
- Mariappan, G., Saha, B.P., Sutharson, L., Singh, A., Garg, S., Pandey, L., Kumar, D. Saudi Pharm. J., 2011, 19(2), 115‒22.
- Saidachary. G., Prasad, K.V., Divya, D., Singh, A., Ramesh, U., Sridhar, B., Raju, B.C. Eur. J. Med. Chem., 2014, 76, 460‒469.
- Wang, X.H., Wang, X.K., Liang, Y.J., Shi, Z., Zhang, J.Y., Chen, L.M., Fu, LW. Chin. J. Cancer, 2010, 29(12), 980‒987.
- Rosiere, C.E., Grossman, M.I. Science, 1951, 113(2945), 651‒653.
- Singh, B.K., Sachan, N., Chawla, P. Curr. Bioact. Comp., 2013, 9(4), 279‒287.
- Harmon, R.E., Geller, B.L., Gupta, S.I., Herbert, M.H., Chitharanjan, D. J. Pharm. Sci., 1970, 59(7), 1031‒1033.
- Basaif, S.A., Hassan, M.A., Gobouri, A.A. Dyes Pigm. 2007, 72, 387‒391.
- Ho, Y.W. Dyes Pigm., 2005, 64, 223‒230.
- Kakiuchi, Y., Sasaki, N., Satoh-Masuoka, M., Murofushi, H., Murakami-Murofushi, K. Biochem. Biophys. Res. Commun., 2004, 320(4), 1351‒1358.
- Tiwari, R.K., Mishra, R.K., Srivastava, S.K., Bahel, S.C. Pesticide Res. J., 1990, 2(1), 24‒27.
- Hadi, V., Koh, Y.-H., Sanchez, T.W., Barrios, D., Neamati, N., Jung, K.W. Bioorg. Med. Chem. Lett., 2010, 20(22), 6854‒6857.
- Datar, P.A., Jadhav, S.R. Int. J. Med. Chem., 2015, 670181.
- Idrees, G.A., Aly, O.M., Abuo-Rahma, G.D., Radwan, M.F. Eur. J. Med. Chem., 2009, 44(10), 3973‒3980.
- Tomkins, P.T., Cooper, K.L., Titchmarsh, S.A., Appleby, P., Webber, D.G. Int. J. Immunopharmacol., 1995, 17(5), 357‒366.
- Siva, K.K., Rajasekharan, A. Inter. J. Res. Pharm. & Chem., 2012, 2(2), 327‒337.
- Devnath, H.P., Islam, M.R. Bangladesh J. Pharmacol., 2010, 5, 30‒33.
- Rahat, K., Imam, U., Alam, M., Sultan, D. J. Bangladesh Parmacol. Soc., 2008, 3, 27‒35.
- Jelich, K., Kraemer, W., Brandes, W., Haenssler, G., Reinecke, P. U.S. Pat. 1987, 4666933 A.
- Kakiuchi, Y., Sasaki, N., Satoh-Masuoka, M., Murofushi, H., Murakami-Murofushi, K. Biochem. Biophys. Res. Commun., 2004, 320(4), 1351‒1358.
- Tripathy, R., Ghose, A., Singh, J., Bacon, E.R., Angeles, T.S., Yang, S.X., Albom, M.S., Aimone. L.D., Herman, J,L., Mallamo, J.P. Bioorg. Med. Chem. Lett., 2007, 17(6), 1793‒1798.
- Gupta, P., Gupta, J.K. Open Chem. J., 2016, 3, 17‒24.
- Tudor, R., Simona, P., Veronica, L., Carmen, C., Raluca, C. Molecules, 2006, 11.
- Min, J.S., Jung, K.K., Bum, S.S., Bo, G.S., Zaesung, N., Hyae, G.C., Kwang-Rok, K., Young, S.S., Hyoung, R.K. Bull. Korean Chem. Soc., 2004, 25(8), 1121.
- Mariappan, G., Saha, B.P., Sutharson, L., Ankit., Garg, S., Pandey, L., Kumar, D. J. Pharm. Res., 2010, 3(12), 2856‒2859.
- Mahmoud, M.M., Ibrahim, S., Abdel, H. J. Chin. Chem. Soc., 2012, 59, 72‒80
- Kumar, S.K., Rajasekharan, A. Inter. J. Res. Pharm. & Chem., 2012, 2(2), 327‒337.
- Parmar, N., Shashikant, T., Rikin, P., Barad, H., Jajda, H., Thakkar, V. J. Saudi Chem. Soc., 2015, 19, 36-41.
- Yamamoto, Y., Kuwahara, T., Watanabe, K. Redox Report, 1996, 2, 333‒338.
- Kazutoshi, W., Yasuhiro, M., Katsuhiko, I., Toshiaki, W., Satoshi, Y., Hiroyoshi, N. Redox Report, 2003, 8.
- Soni, J.P., Sen, D.J., Modh, K.M. J. Appl. Pharm. Sci., 2011, 1(04), 115‒120.
- Mariappan, G., Saha, B.P., Sutharson, L., Haldar, A. Ind. J. Chem., 2010, 49B, 1671‒1674.
- Amir, M., Kumar, S. Ind. J. Chem., 44B, 2005, 2532‒2537.
- Mariappan, G., Saha, B.P., Sutharson, L., Haldar, A. Ind. J. Chem., 2010, 49B, 1671‒1674.
- Georgewill, O.A., Georgewill, U.O., Nwankwoala, R.N.P. The Internet J. Pharmacol., 2009, 7(1), doi: 10.5580/21fc,
- Silva, S.L.D., Comar, J.M., Oliveira, K.M.T., Bezerra, E.R.M., Calgarotto, A.K., Baldosso, P.A., Veber, C.L., Villar, J.A.F.P., Oliveira, A.R.M., Marangoni, S. Inter. J. Quant. Chem., 2008, 108 (13), 2576‒2585.
- Nishiyama, T., Ogawa, M. Acta. Anaesthesiol. Scand., 2005, 49(2), 147‒151.
- Brogden, R.N. Drugs, 1986, 32(4), 60‒70.
- Graham, G.G., Scott, K.F. Inflammopharmacol., 2003, 11, 401– 413.
- Bentur, Y., Cohen, O. J. Toxicol. Clin. Toxicol., 2004, 42, 261– 265.
- Lalit, K., Chandresh, T., Vivek, S. Inter. J. Res. Pharm. & Sci., 2012, 2(2), 13-22.
- Doretto, M.C., Garcia-Cairasco, N., Pimenta, N.J.G., Souza, D.A., Tatsuo, M.A.K.F. Neuro. Report., 1998, 9(10), 2415‒2421.
- Abdel-Aziz, M., Abuo-Rahma, G.E.-D.A., Hassan, A.A. Eur. J. Med. Chem., 2009, 44, 3480–3487.
- Minhaz, U., Tanaka, M., Tsukamoto, H., et al., Free Radic. Res., 1996, 24, 361‒3677.
- Tsujita, K., Shimomura, H., Kawano, H., et al., Am. J. Cardiol., 2004, 94, 481-484.
- Yanagisawa, A., Miyagawa, M., Ishikawa, K., Murota, S. Inter. J. Angiol., 1994, 3, 12‒15.
- Guniz, K., Sevim, R., Habibe, E., Muammer, K., Ekinci, A.C., Vidin, A. Euro. J. Med. Chem., 2000, 35(7-8), 761‒771.
- Ogasawara, K., Inoue, T., Kobayashi, M., Endo, H., Fukuda, T., Ogawa, A. Neurosurgery, 2004, 55, 1060‒1067.
- Kuçukguze, G., Rollas, S., Erdeniz, H., Kiraz, M., Ekinci, A., Cevdet, V.A. Euro. J. Med. Chem., 2000, 35(7-8), 761‒771.
- Oishi, R., Itoh, Y., Nishibori, M., Watanabe, T., Nishi, H., Saeki, K. Stroke, 1989, 20, 1557- 1564.
- Ross, R. N. Engl. J. Med.,1999, 340, 115‒126.
- Evstropov, A.N., Yavorovskaya, V.E., Vorobev, E.S., Khudonogova, Z.P., Medvedeva, S.G., Filimonov, V.D., Prishchep, T.P., Saratikov, A.S. Pharm. Chem. Jour., 1992, 26(5), 426‒430.
- Yuan W.J., Yasuhara, T., Shingo, T., Muraoka, K., Agari, T., Kameda, M., Uozumi, T., Tajiri, N., Morimoto, T., Jing, M., Baba, T., Wang, F., Leung, H., Matsui, T., Miyoshi, Y., Date, I. BMC Neurosci., 2008, 9, 75.
- Takeshi, M., A-Hon, K., Katsushige, T., Zeyu, Q., Tadayoshi, O., Yasuo, K. Shock, 2008, 30(2), 212‒216.
- Hady, S. Br. Med. Jour., 1973, 1(5855), 744.
- Resta, O., Foschino-Barbaro, M.P., Carnimeo, N., Bavoso, P., Picca, V. Respiration, 1984, 46, 121‒127.
- Ramajayam R, Tan KP, Liu HG, Liang PH. Bioorg & Med Chem, 2010; 18: 7849–7854.
- Kees KL, Caggiano TJ, Steiner, K. E, Fitzgerald, J. J, Kates M. J., Christos T. E, Kulishoff J. M, Moore R. D, McCaleb M. L. J. Med. Chem. 1995, 38, 617‒628.
- Kees, K.L., Fitzgerald, J.J., Steiner, K.E., Mattes, J.F., Mihan, B., Tosi, T., Mondoro, D., McCaleb, M.L. J. Med. Chem., 1996, 39, 3920‒ 3928.
- Das N., Verma, A., Shrivastava, P.K., Shrivastava, S.K. Ind. J. chem., 2008, 47B, 1555‒1558.
- Yoshida, H., Sasaki, K., Namiki, Y., Sato, N., Tada, N. Atherosclerosis, 2005, 179, 97‒102.
- Kees, K.L., Caggiano, T.J., Steiner, K.E., Fitzgerald, J.J., Kates, M.J., Christos, T.E., Kulishoff, J.M., Moore, R.D., McCaleb, M.L. J. Med. Chem., 1995, 38, 617‒628.
- Piskarev, A.V., Nesterenko, V.S. Bull. Exp. Bio. Med., 1975, 80(1).
- Dhawan, S., Narang, R., Khatik, G.L., Chopra, H.K., Nayak, S.K. J. Chem. & Pharm. Res., 2016, 8(5), 969‒981.
- Mousa, S.AS., Ishak, E.A., Bakheet, M.E.M., Abu-Shanab, F.A. Elixir Org. Chem., 2015, 89, 36854‒36859.
- Brana, M.F., Gradillas, A., Ovalles, A,G., Lopez, B., Acero, N., Llinares, F., Mingarro, D.M. Bioorg. Med. Chem. 2006, 14(1), 09‒5.
- Castagnolo, D., De Logu, A., Radi, M., Bechi, B., Manetti, F., Magnani, M., Supino, S., Meleddu, R., Chisu. L., Botta, M. Bioorg. Med. Chem., 2008, 16 (15), 8587‒8591.
- Burja, B., Kocevar, M., Polanc, S. Tetrahedron, 2009, 65(42), 8690‒8696.
- Burja, B., Kocevar, M., Polanc, S. Bioorg. Med. Chem., 2010, 18(7), 2375‒2387.
- Akondi, A.M., Kantam, M.L., Trivedi, R., Bharatam, J., Vemulapalli, S.P.B., Bhargava, S.K., Buddana, S.K., Prakasham, R.S. J. Mol. Catal. A: Chem., 2016, 411, 325‒336.
- Bao, X., Wei, S., Zou, L., Song. Y., Qu, J., Wang, B. Tetrahedron: Asymm., 2016, 27(9-10), 436‒441.
- Rao, D.V.N., Prasad, A.R.G., Spoorthy. Y.N., Rao, D.R. Ravindranath, L.K. Ann. Pharm. Fr., 2014, 72(2), 101‒106.
- Kono, M., Proia, R.L. Exp. Cell Res., 2015, 333(2), 178‒182.
- Nakamura, T., Yonesu, K., Mizuno, Y., Suzuki, C., Sakata, Y., Takuwa, Y., Nara, F., Satoh, S. Bioorg. Med. Chem. 2007, 15(10), 3548‒3564.
- Lavergne, K., Bongers, A., Betit, L., Beauchemin, A.M. Org. Lett., 2015, 17(14), 3612‒3615.
- Vereshchagin, A.N., Elinson, M.N., Korshunov, A.D., Egorov. M.P. Mendeleev Commun., 2016, 26(1), 19‒20.
- Chande, M.S., Barve, P.A., Suryanarayan, V. J. Hetero. Chem., 2007, 44(1), 49-53.
- Wu, S., Li, Y., Xu, G., Chen, S., Zhang, Y., Liu, N., Dong, G., Miao, C., Su, H., Zhang, W., Sheng, C. Eur. J. Med. Chem., 2016, 115, 141‒147.
- Amata, E., Bland. N.D., Campbell, R.K., Pollastri, M.P. Tetrahedron Lett., 2015, 56(21), 2832‒2835.
- Ceban, V., Olomola, T.O., Meazza, M., Rios, R. Molecules, 2015, 20, 8574‒8582.
- Li; J.H., Feng, T.F., Du, D.M. J. Org. Chem., 2015, 80(22), 11369‒11377.
- Tu, X-C., Feng, H., Tu, M-S., Jiang, B., Wang, S-L., Tu, S.-J. Tetrahedron Lett., 2012, 53(25), 3169‒3172.
- Khloya, P., Kumar, P., Mittal, A., Aggarwal, N.K., Sharma, P.K. Org. Med. Chem. Lett., 2013, 3(1), 9.
- Kirscuke, K., Luize, G., Schmttz, E.J. Prakt. Chem., 1984, 326(3), 367‒373.
- Chande, M.S., Joshi, R.M. Indian J. Chem., 1999, 38B, 218‒220.
- El-Saraf, G.A., El-Sayed, A.M. Heteroat. Chem., 2003, 14(3), 211‒217.
- Karci, F., Ertan, N. Dyes Pigments, 2002, 55(2), 99‒108.
- Ho, Y.W. Dyes Pigments, 2005, 64(3), 223‒230.
- Khan, R., Uddin, M.I., Alam, M.S., Hossain, M.M., Islam, M.R. Bangladesh J. Pharmacol., 2008, 3(1), 27‒35.
- Huang, Y.-Y., Lin, H.-C., Cheng, K.-M., Su, W.M., Sung, K.-C., Lin, T.-P., Huang, J.-J., Lin, S.-K., Wong, F.F. Tetrahedron, 2009, 65(46), 9592‒9597.
- Ziarati, A., Safaei-Ghomi, J., Rohani, S. Ultrason. Sonochem., 2013, 20(4), 1069‒1075.
- He, Y., Bao, X., Qu, J., Wang, B. Tetrahedron: Asymm., 2015, 26(23), 1382‒1387.
- Mazimba, O., Wale, K., Loeto, D., Kwape, T. Bioorg. Med. Chem., 2014, 22(23), 6564‒6569.
- Trippier, P.C., Zhao, K.T., Fox, S.G., Schiefer, I.T., Benmohamed, R., Moran, J., Kirsch, D.R., Morimoto, R.I., Silverman, R.B. ACS Chem. Neurosci., 2014, 5(9), 823‒829.
- Patel, V.K.V., Sen, D.J. Int. J. Drug Dev. & Res., 2010, 2(1), 203‒210.
- Yang, Z., Wang, Z., Bai, S., Liu, X., Lin, L., Feng, X. Org. Lett., 2011, 13(4), 596‒599.
- Liu, L., Siegmund, A., Xi, N., Kaplan-Lefko. P., Rex, K., Chen, A., Lin, J., Moriguchi, J., Berry, L., Huang, L., Teffera, Y., Yang, Y., Zhang, Y., Bellon, S.F., Lee, M., Shimanovich, R., Bak, A., Dominguez, C., Norman, M.H., Harmange, J.C., Dussault, I., Kim, T,S. J. Med. Chem., 2008, 51(13), 3688‒3691.
- Agejas, J., Ortega, L. J. Org. Chem., 2015, 80(12), 6509‒6514.
- Liu, L., Zhong, Y., Zhang, P., Jiang, X., Wang, R. J. Org. Chem., 2012, 77(22), 10228‒10234.
- Hadi, V., Koh, Y.H., Sanchez, T.W., Barrios, D., Neamati, N., Junga, K.W. Bioorg. Med. Chem., 2010, 20(22), 6854‒6857.
- Bao, X., Wang, B., Cui, L., Zhu, G., He, Y., Qu, J., Song, Y. Org. Lett., 2015, 17(21), 5168‒5171.
- Parekh, N., Thomas, J., John, J., Kusurkar, R., De Borggraeve, W.M., Dehaen, W. J. Org. Chem., 2014, 79(11), 5338‒5344.
- Ma, R., Zhu, J., Liu, J., Chen, L., Shen, X., Jiang, H., Li, J. Molecules, 2010, 15, 3593‒3601.
- Pawar, R.A., Patil, A.A. Ind. J. Chem., 33B, 1994, 156‒158.
- Srivalli, T., Satish, K., Suthakaran, R. Inter. J. Innovative Pharm. Res., 2(4), 2011, 172‒174.
- Kobarkov, K.I., Rybina, I.I., Kelarev, V.I., Koloev, V.K. Chem. Heterocycl. Compd., 39 (6), 2003, 749‒755.
- Naik, C.G., Malik, C,M. Orient J. Chem., 2010, 26(1), 113‒116.
- Althagafi, I., El-Metwaly, N.M., Elghalban, M.G., Farghaly, T.A., Khedr, A.M. Bioinorg. Chem. Appl., 2018, 2727619. doi: 10.1155/2018/2727619
- Yoon, S., Choi, B., Rahman, M.M., Kumar, S., Kabir, S.M.M., Koh, J. Materials., 2019, 12(24), 4209. doi: 10.3390/ma12244209
- Zhang, Y., Zhao, K.T., Fox, S.G., Kim, J., Kirsch, D.R., Ferrante, R.J., Morimoto, R.I., Silverman, R.B. J Med Chem., 2015, 58(15), 5942–5949.
- Pei, L., He, S., Gao, J., Liao, H., Gao, H. Polymers, 2017, 9(7), 262. doi: 10.3390/polym9070262
- Yepremyan, A., Mehmood, A., Brewer, S., Barnett, MM., Janesko, B.G., Akkaraju, G., Simanek, E.E., Green, KN. RSC Adv., 2018, 8(6), 3024–3035.
- Amata, E., Bland, N.D., Campbell, R.K., Pollastri, M.P. Tetrahedron Lett., 2015, 56(21), 2832–2835.
- Mochona, B., Jackson, T., McCauley, D., Mazzio, E., Redda, K.K. J. Heterocycl. Chem., 2016, 53(6), 1871–1877.
- Sweeney, N.L., Lipker, L., Hanson, A.M., Bohl, C.J., Engel, K.E., Kalous, K.S., Stemper, M.E, Sem, D.S., Schwan, W.R. Antibiotics, 2017, 6(1), 4. doi: 10.3390/antibiotics6010004
- Mariappan, G., Saha, B.P., Sutharson, L., Singh, A., Garg, S., Pandey, L., Kumar, D. Saudi Pharm. J., 2011, 19(2), 115–122.
- Sivakumar, K.K., and Rajasekaran, A. J. Pharm. Bioallied. Sci., 2013, 5(2), 126–135.

