- soils; Diazinon; Chlorpyrifos; clay; sorption constant (Kd); Langmuir and Freundlich models.
Copyright (c) 2021 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
Intensive application of pesticides in the agricultural sector and for domestic purposes has resulted in increased usage over the years. Pesticides are used to control pest, diseases and weeds in agricultural and urban areas, but their persistence in the environment has resulted in human poisoning, health risk problem and environmental pollution due to their ability to permeate the soil surface, groundwater systems and water surface bodies.
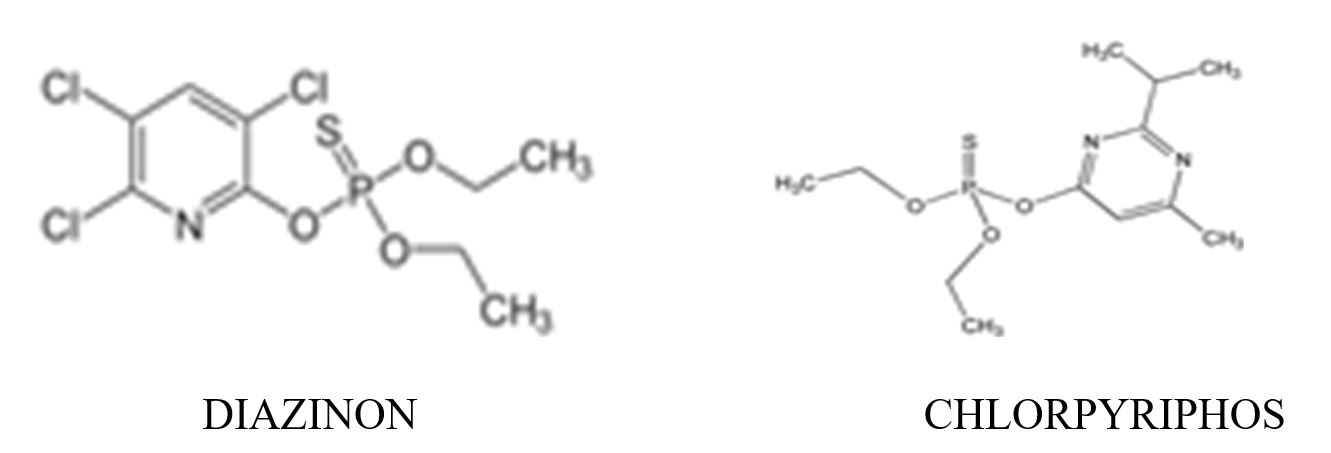
Sorption of two organophosphorus pesticides: Diazinon and Clorpyriphos was studied in a soil (S) and in soil modified with clay addition. Soil Alhue, VI region, Chile, was spiked with 1% of either Montmorillonite (M) or Kaolinite (K). In addition, organic matter (OM), in the soil was eliminated and this soil was spiked again, with 1% of both clays.
Batch sorption and kinetics experiments were conducted to obtain the retained amounts onto the soil samples. Pesticides were quantified by high performance liquid chromatography (HPLC).
The results showed that for both pesticides the sorption order is: S-M ˃ S-K > S. For soil without OM the order is the same, although the adsorption was lower. The adsorption isotherms were expressed by the Langmuir and Freundlich models. The negative Gibb’s free energy change (ΔG°) values obtained suggest that the adsorption of both pesticides, is an exothermic process.
Clay addition to the soil, increased the adsorption processes, generating an increase in the amount of pesticide retained in the soil and partially avoiding possible contamination of the aquifers. The difference found in relation to the behavior of both clays on the soil, can be explained considering its structure and its octanol-water partition coefficient (kow).
References
2. P. M. Huang, R. B. McKercher, Soil Science. 138, 20, (1984).
3. G. J. Welhouse, W. F. Bleam, Environ. Sci. Technol. 26, 959, (1992b).
4. G. J. Welhouse, W. F. Bleam, Environmental Science & Technology. 27, 594, (1993a).
5. J. Pignatelo, B. Xing, Environmental Science of Technology. 30, 1, (1996).
6. G. Singh, W. F. Spencer, M. M.Cliatb, M. Th. Van Genubten, Journal of Environmental Quality. 19, 520, (1990).
7. E. Barriuso, U. Baer, R. Calvet, Journal of Environmental Quality, 21, 359, (1992).
8. R. D. Wauchope, J.B.H. Yeh, R. Lindres, K. Kloskowski, B. Tanaka, J. Rubin, Pesticide Management Science. 58, 419, (2002).
9. T. Berglof, W. C.. koskinen, M. J. Duffy, k. A. Norberg, H. Kylin, Journal of Agricultural and Food Chemistry. 51, 3598, (2003).
10. B. S. Ismail, K.E. Ooi, Journal of Environmental Biology. 33, 573, (2012).
11. J. H. Cao, H. M. Guo, L. Zhu, H. Jiang, H. Yang, Chemosphere. 70, 2127, (2008).
12. X. Li, Q. Zhou, S. Wei, W. Ren, X. Sun, Geoderma. 160, 347, (2011).
13. N. Singh, Journal of Agricultural and Food Chemistry. 50, 6434, (2002).
14. G. W. Bailey, J. L. White, Journal of Agricultural and Food Chemistry. 2, 324, (1964).
15. M. H. B. Hayer, Residue Review. 32, 131, (1970).
16. M. Arienzo, A. Buondonno, Toxicological & Environmental Chemistry 39, 193, (1993).
17. B. M. Jenks, F. W. Roeth, A. D. Martin, D. L. Mccallister, Weed Science. 46, 132, (1998).
18. M. Beckbolet, O. Yernigun, T. Yucel, Water air soil Pollut. 111, 75, (1999).
19. E. M. Murphy, J. M. Zachara, S. C. Smith, J. L. Phillips, Science and Total Environment. 1, 17-118, 413, (1992).
20. S. Baskaran, N. S. Bolan, A. Rahman, R. W. Tillman, J. Agric. Res. 39, 297, (1996).
21. B. Singh, A. Walker, Microbiol. Rev. 30, 428, (2006).
22. P. Montuori, S. Aurino, A. Nardone, T. Cirillo, M. Triassi, Environ. Sci. Pollut. Res. Int., 22, 8629, (2015).
23. R. Ahmad, P.N. Nelson, R. S. Kookana, J. Soil Sci. 57, 883, (2006).
24. L. Nemeth-kunda, G. Fûleky, G. Morovjan, P. Csokam, Chemosphere. 48, 545, (2002).
25. M. C. Fernández, L. Cox,M. C. Hermosin, J. Cornejo, Pest. Manag. Sci. 59, 545, (2003).
26. D.P. Oliver, R. S. Kookana, B. Quintana, J. Agric. Food Chem. 53, 6420, (2005).
27. A. Sadzawka, M. A. Carrasco, R. Grez, M. L. Mora, H. Flores, A. Neuman, Métodos de Análisis de Suelos. Instituto de Investigaciones Agropecuarias (INIA). Serie Actas INIA 34, 59, 2006.
28. Servicio agrícola y ganadero de Chile (2012). Informe de ventas de plaguicidas de uso agrícola en Chile, año 2012. http://www.sag.cl/sites/default/files/declaracion_de_venta_de_plaguicidas_ano_2012.pdf
29. Feng-Chin Wu, Ru-Ling Tseng, Ruey-Shin Juang . Chemical Engineering Journal. 150, 366, (2009).
30. OECD. Guidelines for testing of chemicals, Section 1 (106):Adsorption-Desorption using batch equilibrium method in soils. Environmental Health and Safety Division, Organisation for Economic Co-operation and Development (OECD), Environment. Directorate, Paris, France. 2000
31. X. Chen, Information 6, 14, (2015).
32. M. R. Shariff, International Journal of Engineering Research and Development 1, 55, (2012).
33. K. S. Ahmad, N. Rashid, M. F. Nazar, S. Tazaiyen, Journal of the Chemical Society of Pakistan 34-35, 1017, (2014).
34. I. Langmuir, J. Am. Chem. Soc. 38, 2221–2295 (1916).
35. A. O. Dada, A. P. Olalekan, A. M. Olatunya, O. Dada, Journal of Applied Chemistry. 3, 38, (2012).
36. J. Porta, M. Lopez – Acevedo, R. Porch, Edafología: uso y protección de suelos. 3ed. 272, 2014.
37. Y. Liu, Z. Xu, X. Wu, W. Gui, G. Zhu., Journal of Hazardous Materials. 178, 462, (2010).
38. D. Pal, S. K. Maiti, Environ Sci Pollut Res Int, 25, 12464, (2018).
39. F. Sadegh-Zadeh, S. A. Wahid, D. Omar, R. B. Othman, B. J. Seh-Bardan, Soil and sediment contamination, 20, 387, (2011).
40. H. J. Turin, R. S. Bowman, Journal of environmental quality, 26, 1282-1287 (1997).
41. S. D. Nelson, W. J. Farmer, J. Letey, C. F. Williams, Journal of environmental quality, 29, 1856-1862 (2000).
42. B. Von Oepen, B. W. Kördel, W. Klein, Chemosphere 22, 285-304 (1991).
43. C. H. Giles, T. H. MacEwan, S. N. Nakhwa, D. Smith, Journal of the Chemical Society. 3, 3973-3993 (1960).
44. J. P. Aguer, L. Cox, C. Richard, M. C. Hermosin, J. Cornejo, Journal of environmental science and health part B, 35, 725-738 (2000).
45. C. Dolaptsoglo, D. G. Karpouzas, U. Menkissoglu-Spiroudi, L. Eleftherohorinos, E. A. Voudrias, Journal of environmental quality. 36, 1793-1802 (2007).
46. F. A. Vega, Spanish journal of soil Sciencie. 1, 20-37 (2011).
47. A. F. Chamorro, R. D. Sanchez, Revista de Ciencias. 16, 145-160 (2012).
48. H.M.F. Freundlich, J. Phys. Chem. 57, 385–471 (1906).
49. Y. Long You, X. Wu, N. Shao, H. Fang, H. Zhan, J. Quan Yu, Environ. Pollut. 141:428-433 (2005).
50. O. P. Bansal. Journal of Applied Sciences and Environmental Management 14, 155-158 (2010).
51. E. H. Pérez, C. A. Arboleda, Suelos Ecuatoriales, 46, 59-66 (2016).
52. S. Gebremariam, M. Beutel, D. Yonge, M. Flury, J. Harsh, Rev Environ Contam Toxicol. 215, 123-75 (2012).
53. B. Gevao, K.T. Semple, K. C. Jones K.C. 2000. Environmental Pollution. 108, 3-14 (2000).
54. J. Cornejo, R. Celis, I. Pavlovic, M. A. Ulibarri. A review. Clay Minerals. 43, 155–175 (2007).
55. V. C. Farmer, M. M. Mortland., Journal of the Chemical Society A. 3, 344 – 351 (1966).
56. G. Lagaly, Applied Clay Science, 18, 205 – 209 (2001).
57. J. J. Hasset, W. L. Banwart. The sorption of nonpolar organics by soils and sediments. En: Shawhney, B. L. y Brown, K. (Eds.), Reaction and movement of organic chemicals in soils. 31-44, 1989.
58. W. C. Koskinen, S. S. Harper. The retention process: mechanisms. En: Cheng, H. H. (Ed.), Pesticides in the soil environment: Processes, Impacts, and Modeling. Soil Science Society of America, Inc. Madison, Wisconsin. 51-78, 1990.
59. C. T. Chiou. Partition and adsorption of organic contaminants in environmental systems. John Wiley and Sons. Hoboken, New Jersey. 150-168. 2002
60. P. Sollins, P. Homann, B. A. Caldwell, Geoderma. 74, 65–105 (1996).
61. X. Ma, X. W. J. Bruckard, R. Holmes, Instituto J. Miner. 93, 54-58 (2009).
62. M. R. Seger, G. E. Maciel, Environ. Sci.Technol. 40, 791-796 (2006).
63. A. Kausar, M. ,Iqbal, A. Javed, K. Aftab, Z. Nazli, H. Bhatti, S. Nouren, A review. Journal of Molecular Liquids. 256, 395–407 (2017).
64. S. Rihs, A. Gontier, E. Lascar, A. Biehler, M. P. Turpault, Applied Clay Science. 147: 128–136 (2017).