SYNTHESIS AND CHARACTERIZATION OF COPPER NANOPARTICLES SUPPORTED IN CARBON NANOTUBES MULTIPLE WALLS PREPARED BY CLD AND SMAD
- Copper nanoparticles,
- Carbon nanotubes,
- Electron diffraction,
- Transmission electron microscopy
Copyright (c) 2021 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
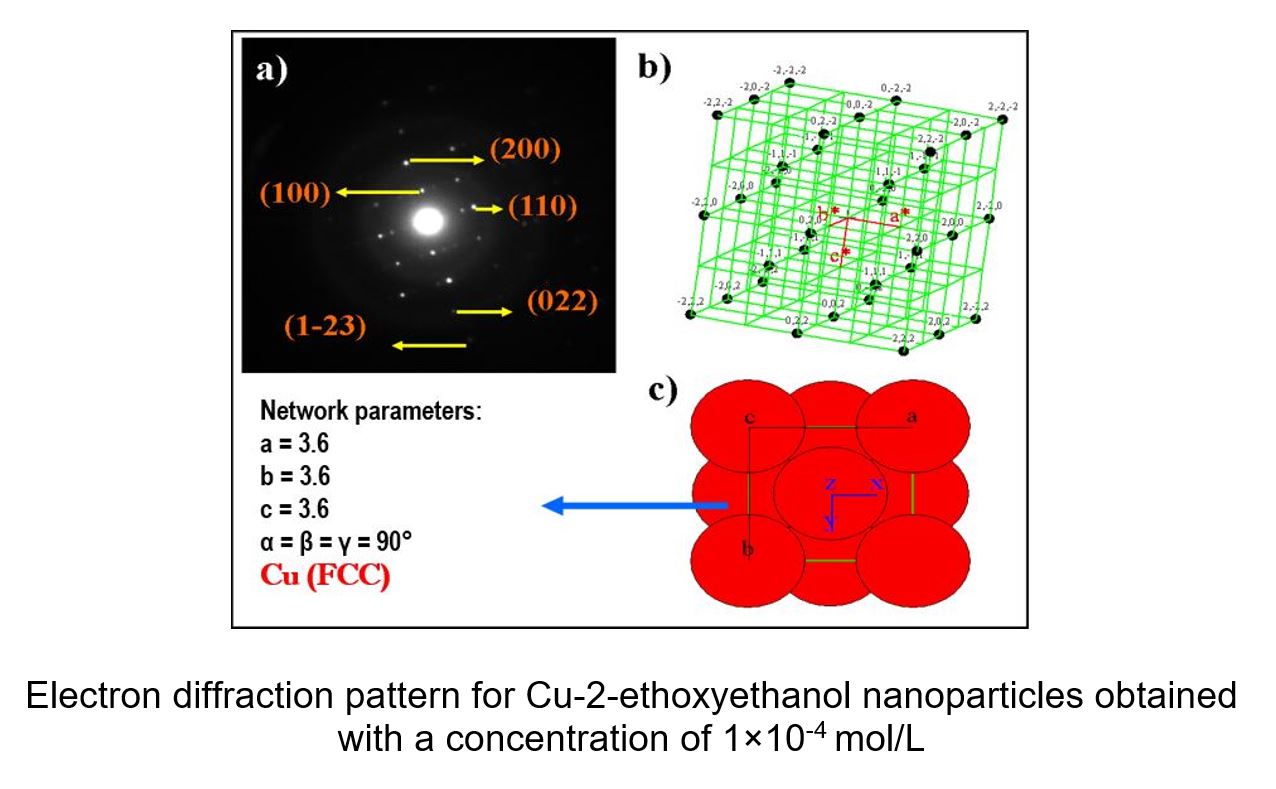
The synthesis and characterization of copper nanoparticles supported in carbon nanotubes is reported. Copper nanoparticles were obtained by the CLD (chemical liquid deposition) method consisting of reduced pressure evaporation of metals and subsequent low-temperature condensation (77K) of these vapors, with organic solvent vapors. From the reactions obtained by this method, copper colloids of concentrations 10-3 and 10-4 were synthesized in the organic solvents 2-propanol, 2-mercaptoethanol, 2-methoxyethanol and 2-ethoxyethanol. The support of copper nanoparticles in the carbon nanotubes was made by SMAD method with 24 hours under agitation and nitrogen atmosphere before collecting, obtaining copper nanoparticle solids supported in carbon nanotubes by evaporating the solvent either 2-mercaptoethanol or 2-ethoxyethanol. Colloidal and solid dispersions were characterized by diverse techniques, for colloidal dispersions UV-Vis spectrophotometry, electrophoretic mobility, electron transmission microscopy (TEM), electron diffraction and stability over time was used. Far-medium infrared spectroscopy (FT-IR) and thermogravimetry (TGA) were used for solids. These analyses show that Cu colloids are relatively stable in the solvents used, the most stable dispersion was synthesized in 2-mercaptoethanol and the less stable is synthesized in 2-methoxyethanol, which is demonstrated in UV spectra. By TEM, particle sizes were studied, between 5.2 and 17.2 nm. Electron diffraction confirms the presence of copper. Analysis of active solids shows in the case of FTIR that solvents are incorporated into copper particles, and in the case of thermograms it is shown that the solids synthesized in the solvent had greater thermal stability 2-mercaptoethanol. It was impossible to obtain electron diffraction from the copper nanoparticles visualized because they are incorporated in the multiwall nanotubes.
References
2. Haynes WM 2016 Thermal and physical properties of pure metals. In CRC Handbook of Chemistry and Physics, 97th edn,pp.12-218. Boca Raton, FL CRC Press.
3. Subramaniam C, Yamada T, Kobashi K,Sekigushi A. Futaba DN, Yumura M and Hata K. One hundred fold increase in current carrying capacity in a carbon nanotube -copper composite. Nat Commun, 4: 2202 (2013). doi 10.1038/ncomms3202
4. International Copper Study Group.The World Copper Factbook.Portugal: International Copper Study Group, (2014).
5. Sabramaniam C, Yasuda Y, Takeya S, Ata S, Nichisawa A, Fucaba O, Yamada T and Hata K. Carbon-nanotube copper exhibiting metal-like thermal conductivity and silicon-like thermal expansion for efficient cooling of electrons. Nanoscale 6: 2669-2674, (2014). doi:10.1039/c3nr05290g)
6. Santos-Hernández D, González-García M.B and Costa-García A. Metal‐Nanoparticles Based Electroanalysis. Electroanalysis 14, issue 18: 1225-1235, (2014). https://doi.org/10.1002/1521-4109(200210)14:18<1225::AID-ELAN1225>3.0.CO;2-Z
7. Cárdenas Galo and Lillo Viviana. Synthesis & characterization of Co-Ni colloids prepared in nonaqueous solvents. J. Chil. Chem. Soc Vol 52: 1182-1185, (2007). doi.org/10.4067/S0717-97072007000200014.
8. Blackborow, J. & Young, D., “Metal Vapour Synthesis in Organometallic Chemistry”, Springer- Verlag, Berlín pag. 14, (1979).
9. Aryasomayajula L and Wolter K. Carbon nanotube composites for electronic packaging applications: a review. J. Nanotechnol. 296517: 1-6, (2013). doi:10.1155/2013/296517
10. Curtin WA and Sheldon BW. CNT-reinforced ceramics and metals. Mater. Today 7: 44-49, (2004). doi:10.1016/S1369-7021(04)00508-5
11. Neubauer E, Kitzmantel M, Hulman M and Angerer P. Potential and challenges of metal matrix-composites reinforced with carbon nanofibers and carbon nanotubes. Compos. Sci. Technol. 70: 2228-2236, (2010). doi:10.1016/j. compscitech.2010.09.003
12. Silvestre N. State-of-the-art review on carbon nanotube reinforced metal matrix composites. Int. J. Compos. Mater. 3(A): 28– 44, (2013).
13. Azarniya A, Safavi M, Sovizi S, Azarniya A, Chen B, Madaah Hosseini H and Ramakrishna S. Metallurgical challenges in carbon nanotube reinforced metal matrix nanocomposites. Metals 7: 384, (2017). doi:10.3390/met7100384
14. Azarniya A, Azarniya A, Sovizi S, Hosseini HRM, Varol T, Kawasaki A and Ramakrishna S. Physicomechanical properties of spark plasma sintered carbon nanotube-reinforced metal matrix nanocomposites. Prog. Mater. Sci. 90: 276-324 (2017). doi: 10.1016/j.pmatsci.2017.07.007
15. Bakir M and Jasiuk I. Novel metal-carbon nanomaterials: a review on covetics. Adv. Mater. Lett. 8: 884-890, (2017). doi:10.5185/amlett. 2017.1598
16. Jayathilaka WADM, Chinnappan A and Ramakrishna S. A review of properties influencing the conductivity of CNT/Cu composites and their applications in wearable/flexible electronics. J. Mater. Chem. C 5: 9209-9237, (2017). doi:10.1039/ C7TC02965A
17. Singh A, Ram Prabhu T, Sanjay AR and Koti V. An overview of processing and properties of Cu/ CNT nano composites. Mater. Today Proc. 4: 3872-3881, (2017). doi:10.1016/j.matpr.2017.02.286
18. Janas D and Liszka B. Copper matrix nanocomposites based on carbon nanotubes or graphene. Mater. Chem. Front. 2: 22-35, (2018). doi:10.1039/C7QM00316A
19. Arnaud C, Lecouturier F, Mesguich D, Ferreira N, Chevallier G, Estournes C, Weibel A and Laurent C. High Strength-high conductivity double-walled carbon nanotube-copper composite wires. Carbon 96: 212-215, (2016). doi:10.1016/j.carbon.2015.09.061
20. Melendrez M.F, Cárdenas G, Diaz-V J, Cruzat C and Arbiol J. Synthesis and aggregation study of tin nanoparticles and colloids obtained by chemical liquid deposition. Colloid Polym Sci 287: 13-22, (2009). https://doi.org/10.1007/s00396-008-1950-7
21. Cárdenas G. Chemical reactions at nanometal particles J. Chil. Chem. Soc. 50 N° 3: 603-612, (2005).
22) Tello A, Cárdenas G, Häberle P and Segura R.A. The synthesis of hybrid nanostructures of gold nanoparticles & carbon nanotubes & their transformation to solid carbon nanorods. Carbon 46, issue 6: 884-889, (2008). https://doi.org/10.1016/j.carbon.2008.02.024
23. Turkevich J and Kim G. Palladium: Preparation and Catalytic Properties of Particles of Uniform Size. Science 169, issue 3948:873-879, (1970). doi: 10.1126/science.169.3948.873
24. Creigthon J. A and Eadon D.G. Ultraviolet–visible absorption spectra of the colloidal metallic elements. J. Chem Soc. Faraday .Trans 87: 3881-3891, (1991). https://doi.org/10.1039/FT9918703881
25. Cárdenas G, Leon Y, Moreno Y and Peña O. Synthesis and properties of NiSn colloids using different metal ratios by CLD. Collid Polym Sci 284, N°6: 644-653, (2006). doi: 10.1007/s00396-005-1429-8
26. i) Cárdenas G, Oliva R and Gielsing M. Copper colloids from no-aqueous solvents. Part IV Bol. Soc. Chil. Quim 38, (1993).
ii) Cárdenas G, Matsuo K and Klabunde K.J .Tin colloids and metal-metal oxide films prepared by chemical liquid deposition. J. Org. Chem 47, 843 (1982).
iii) Cárdenas G and Oliva R. Colloids & films of Cu; Ag & Au from non-aqueous solvent. Part VI Bol. Soc. Chil. Quim.; 38, 301-308 (1993).
27. Sainsbury T, Stolarczyk J and Fritzmaurice D. An experimental & theoretical study of the self-assembly of gold nanoparticles at the surface of functionalized multiwalled carbon nanotubes. J. Phys. Chem 109: 16325 (2005).