OPTIMIZING THE STRUCTURE OF AMPHIPHILIC INVERTIBLE POLYMERS (AIPs) MADE OF PEGS AND FATTY COMPOUND SEGMENTS TO OBTAIN A SINGLE CRITICAL MICELLE CONCENTRATION

- Biopolyesters,
- Amphiphilicity,
- Fatty compounds,
- PEGs,
- inverted micelles
Copyright (c) 2020 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
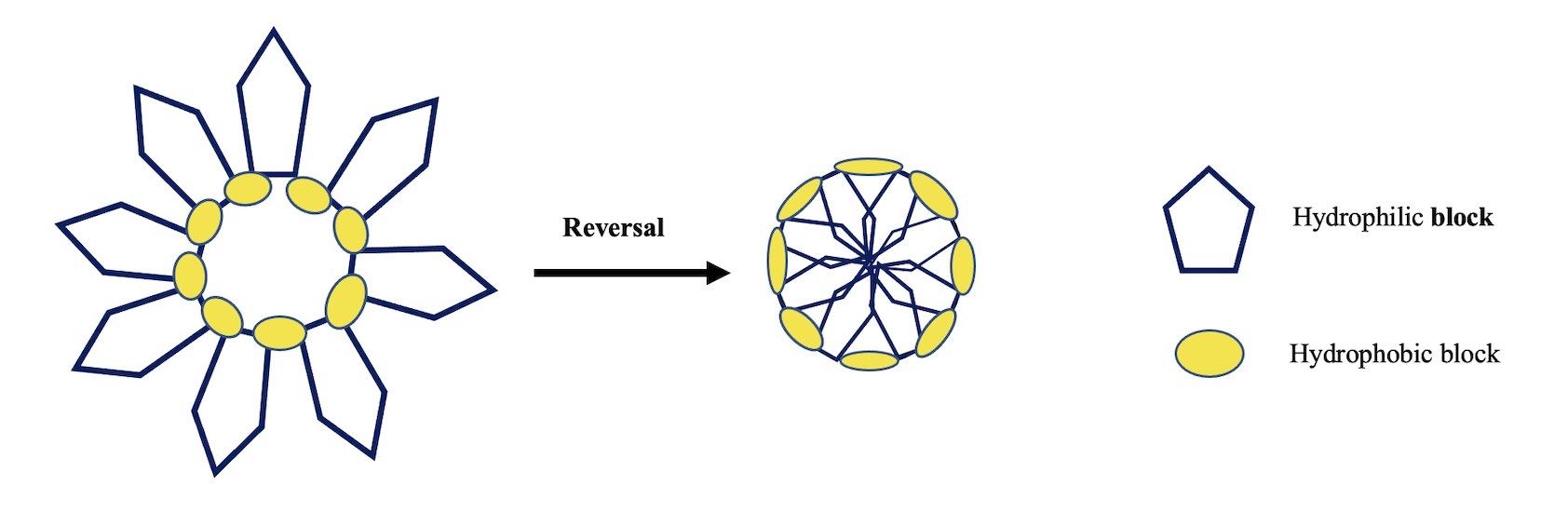
Amphiphilic biopolyesters containing hydrophilic segments (PEGs) and hydrophobic blocks (silicon fatty from a castor oil derivative) showed the ability to self-assembly in inverted micelles. Due to their capability to form also direct micelles, these biopolyesters could be classified as amphiphilic invertible polymers AIPs. The micellar concentrations CMC and ICMC corresponding to the direct and inverted micelles respectively precisely correlated with PEG length. The methodology used in this investigation allowed to determine the PEG length needed to obtain the adequate structural biopolyester able to self-assembly in direct and inverted micelles from a unique concentration. Inverted micelle diameters, determined by DLS analysis, increased as the molecular polarity of the biopolyesters decreased. No individual inverted micellar were observed by TEM technique due to the concentration change during the sample preparation, however micellar macromolecular aggregations were revealed.
References
- S. Van Vlierberghe, P. Dubruel, E. Schacht, Biomacromolecules 12, 1387,( 2011).
- R. Zarzycki, Z. Modrzejewska, K. Nawrotek, U. Lek, Ecol. Chem. Eng. S. 17, 117, (2010).
- T. Zheng, Y. Liang, S. Ye, Z. He, Biosyst. Eng. 102, 44, (2009).
- F. Brandl, F. Kastner, R. M. Gschwind, T. Blunk, J. Teßmar, A. Göpferich, J. Control. Release 142, 221, (2010).
- N. S. Malik, M. Ahmad, M. U. Minhas, PLoS One 12, 1, (2017).
- N Katir, D. Andrade,M. Dahrouch, E. Diaz, N. Gatica, D. Hourlier, N. Reyes, M. Zarraga, J. Chil. Chem. Soc. 1, 2784, (2016).
- M. R. Shahid Bashir, Y. Y. Teo, S. Ramesh, K. Ramesh, A. A. M. Rizwan, J. Chil. Chem. Soc. 64, 2, (2019).
- B. A. Laurent, S. M. Grayson, Polym. Chem. 3, 1846, ( 2012).
- L. Yin, Y. Chen, Z. Zhang, Q. Yin, N. Zheng, J. Cheng, Macromol. Rapid Commun. 36, 483, (2015).
- N. M. Correa, J. J. Silber, R. E. Riter, N. E. Levinger, Chem. Rev. 112, 4569, (2012).
- G. N. Smith, P. Brown, S. E. Rogers, J. Eastoe,Langmuir 29, 3252, (2013).
- S. Taokaew, M. Ofuchi, T. Kobayashi, Materials (Basel) 12, 1160, (2019).
- L. P. Sze, H. YinLi, K. L. A. Lai, S. F. Chow, Q. Li, K. W. KennethTo, T. N. T. Lam, W. Y. T. Lee, Colloids Surfaces B Biointerfaces 184, 110554, (2019).
- X. Zhang, N. Liang, X. Gong, Y. Kawashima, F. Cui, S. Sun, Colloids Surfaces B Biointerfaces 177, 11, (2019).
- R. G. Thomas, M. J. Moon, J. H. Kim, J. H. Lee, Y. Y. Jeong, PLoS One 10, 1, (2015)
- B. Zhang, H. Zhang, Y. Li, J. N. Hoskins, S. M. Grayson, ACS Macro Lett. 2, 845, (2013).
- D. Abdelhamid, H. Arslan, Y. Zhang, K. Uhrich, Polym. Chem. 5,1457,( 2014)
- G. B. Behera, B. K. Mishra, P. K. Behera, M. Panda, Adv. Colloid Interface Sci. 82, 1, (1999).
- T. Yang, W. Li, X. Duan, L. Zhu, L. Fan, Y. Qiao, H. Wu, PLoS One 11, 1, (2016).
- H. C. Andrés, F. Olea, J. Villena, A. Moller, R. Martínez, J. Chil. Chem. Soc. 64, 4437, (2019).
- J. J. Silber, R. D. Falcone, N. M. Correa, M. A. Biasutti, E. Abuin, E. Lissi, P. Campodonico, Langmuir 19, 2067, (2003).
- U. Anand, C. Jash, S. Mukherjee, J. Colloid Interface Sci. 364,. 400, (2011).
- A. Harada, K. Kataoka, Polym J 50, 1, (2017).
- S. Yi, F. Dai, C. Zhao, Y. Si, Sci. Rep 7, 1, (2017).
- M. S. Akhter, Colloids Surfaces A Physicochem. Eng. Asp. 150, 25, (1999).
- C. Reichardt and T. Welton, Solvents and Solvents Effects in Organic Chemistry, Fourth edi. Germany: Wiley-VCH, 2011.
- A. Voronov, Biomacromolecules 13, 2537, (2012).
- A. Maran, M. J. Yaszemski, A. Kohut, A. Voronov, materials 9, 10, (2016).
- S. Ramadurai, A. Kohut, N. Kumar, O. Zholobko, J. Colloid Interface Sci. 542, 483, (2019).
- D. andrade, C. Moya, F. Olate, N. Gatica, S. Sánchez, E. Díaz, E. Elgueta, M. Parra, M. Dahrouch,
- RSC Adv., 6, 38505 (2016).
- J. W. Bae, E. Lee, K. M. Park, K. D. Park Macromolecules, 42, 3437, (2009).
- F. Bougard, C. Giacomelli, L. Mespouille, R. Borsali, P. Dubois, R. Lazzaroni, Langmuir 24,8272, (2008).
- Q. Cui, F. Wu, E. Wang, J. Phys. Chem. B 115, 5913, (2011).
- C. L. Salcedo, A. M. Bouchet, M. A. Nazareno, E. A. Disalvo, M. A. Frias, Colloids Surfaces B Biointerfaces, 113, 243, (2014).
- A. M. Bouchet, M. A. Frías, F. Lairion, F. Martini, H. Almaleck, G. Gordillo, E. A. Disalvo, Biochim. Biophys. Acta - Biomembr. 1788, 918, (2009).
- L. Chen, T. Ci, L. Yu, J. Ding, Macromolecules 48, 3662, (2015).
- B. Obermeier, H. Frey, Bioconjug. Chem. 22, 436, (2011).
- N. Katir, A. El Kadib, M. Dahrouch, A. Castel, N. Gatica, Z. Benmaarouf, P. Riviere, Biomacromolecules 10, 850, (2009).

