SYNTHESIS, CRYSTAL STRUCTURE AND HIRSHFELD SURFACE ANALYSIS OF A NEW COORDINATION POLYMER: STRONTIUM BENZILATE
- Benzilate,
- coordination polymer,
- X-ray diffraction,
- hydrogen bonds,
- Hirshfeld surface
Copyright (c) 2021 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
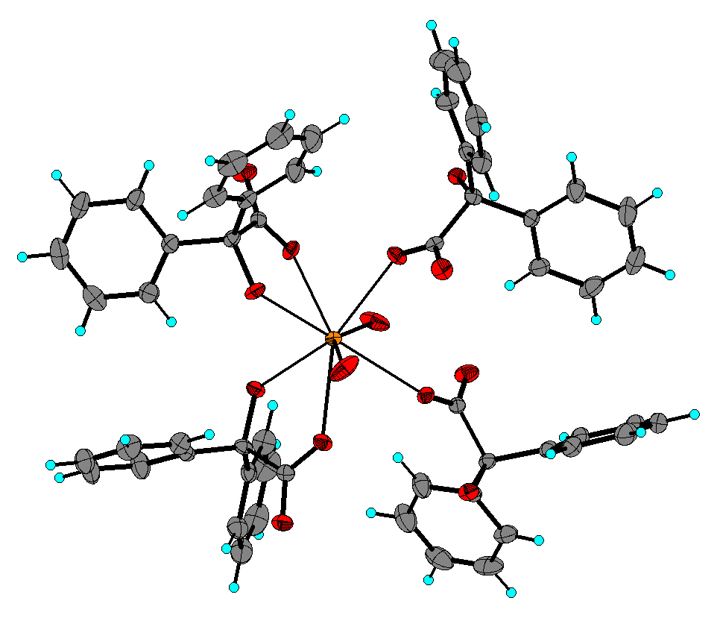
A novel coordination polymer was synthesized from strontium carbonate and benzylic acid in aqueous solution and is formulated as Sr[(C6H5)4(COCOO)2·2H2O]. This compound was characterized by FTIR spectroscopy and powder X-ray diffraction. The crystal structure was determined by single-crystal X-ray diffraction. The complex crystallizes in the monoclinic P21/n space group, with unit cell parameters a = 15.0224(9) Å, b = 7.5038(6) Å, c = 25.000(2) Å, b = 94.764(2)º, V = 2808.4 Å3, Z = 4. In the structure, the metallic ion is coordinated to eight oxygen atoms, six from benzilate molecules, and two from water molecules, forming a distorted tetragonal antiprism. One of the benzilates is coordinated to the metal in a monodentate fashion (carboxylate only), while the other benzilate molecule does it in the bidentate from carboxylate and hydroxide. Strontium ions form infinite zig-zag chains along the [010] direction, which form a three-dimensional network via O--H···O hydrogen-bond interactions between the coordinated water molecules and the O atoms of the carboxylate groups. The intermolecular interactions were analyzed using Hirshfeld surface analysis.
References
2. K.T. Mahmudov, M. N. Kopylovich, F. C. Guedes da Silva, A. J. L. Pombeiro, Coord. Chem. Rev. 345, 54 (2017).
3. E. Bermejo, R. Carballo, A. Castiñeeiras, A. B. Lago, Coord. Chem. Rev. 257, 2639 (2013).
4. Gielen M., Tiekink E.R.T. (Eds.), 2005. Metallotherapeutic drugs and metal-based diagnostic agents: The use of metals in medicine, John Wiley & Sons, Chichester.
5. Jones C., Thornback J., 2007. Medicinal applications of coordination chemistry, The Royal Society of Chemistry, Cambridge.
6. A. F. Alshamrani, T. J. Prior, B. P. Burke, D. P. Roberts, S. J. Archibald, L. J. Higham, G. Stasiuk, C. Redshaw, Inorg. Chem. 59, 2367 (2020).
7. K. S. Lee, K. A. Frey, R. A. Koeppe, A. Buck, G. K. Mulholland, D. E. Kuhl, J. Cereb. Blood Flow Metab. 16, 303 (1996).
8. D. G. Drescher, T. P. Kerr, M. J. Drescher, Brain Res. 845, 199 (1999).
9. S. Yamada, Y. Ito, S. Nishijima, K. Kadekawa, K. Sugaya, Pharmacol. Ther. 189, 30 (2018).
10. C. M. Timperley, M. Bird, S. J. Gore, C. D. Lindsay, H. Rice, J. E. H. Tattersall, C. L. Whitmore, A. C. Green, Toxicol. Lett. 325, 67. (2020).
11. Ferreira-Vieira H.T, Guimaraes I.M., Silva F.R., Flavia F.M., 2016. Alzheimer's disease: targeting the cholinergic system, Curr. Neuropharmacol. 14, 101-115.
12. B. Yiğit, M. Yiğit, D. B. Celepci, Y. Gök, A. Aktaş¸ M. Aygün, P. Taslimi, I. Gülcin, ChemistrySelect, 3, 7976 (2018).
13. A. J. Mora, A. N. Fitch, B. Ramirez, G. E. Delgado, M. Brunelli, J. Wright, Acta Cryst. B, 59, 378 (2003).
14. L. S. Rojas, B. M. Ramírez, A. J. Mora, G. E. Delgado, G. Díaz de Delgado, Acta Cryst. E, 59, m647 (2003).
15. E. Halevas, A. Hatzidimitriou, M. Bertmer, A. A. Vangelis, A. Antzara, C. Mateescu, A. Salifoglou, Cryst. Growth Des. 14, 4041 (2014).
16. U. S. Soumya Mol, R. Drisya, P. R. Satheesh Chandran, M. R. Sudarsanakumar, S. Suma, P. K. Sudhadevi Antharjanam, J. Mol. Struct. 1125, 73 (2016).
17. R. Carballo, B. Covelo, E. M. Vázquez-López, E. García-Martínez, A. Castiñeiras, J. Niclos, Z. Anorg. Allg. Chem. 631, 785 (2005).
18. Y. Qiu, K. Wang, Y. Liu, H. Deng, F. Sun, Y. Cai, Inorg. Chim. Acta, 360, 1819 (2007).
19. P. Halder, B. Chakraborty, P. R. Banerjee, E. Zangrando, T. K. Paine, CrystEngComm, 11, 2650 (2009).
20. R. Carballo, B. Covelo, N. Fernández-Hermida, A. B. Lago, E. M. Vázquez López, J Chem Cryst. 41, 1949 (2011).
21. C. Janiak, Dalton Trans. 2781 (2003).
22. S. Kitagawa, R. Kitaura, S. Noro, Angew. Chem. Int. Ed. 43, 2334 (2004).
23. J. Q. Liu, Z. D. Luo, Y. Pan, S. Kumar, M. Trivedi, A. Kumar A., Coord. Chem. Rev. 406, 213145 (2020).
24. J. Vallejos, I. Brito, A. Cárdenas, M. Bolte, S. Conejeros, P. Alemany, J. Llanos, Polymers, 8, 46 (2016).
25. J. Vallejos, I. Brito, A. Cárdenas, M. Bolte, J. Llanos, M. López-Rodríguez, V. Lavín, I. R. Martín, Inorg. Chem. Commun. 39, 14 (2014).
26. J. Vallejos, I. Brito, A. Cárdenas, J. Llanos, M. Bolte, M. López-Rodríguez, J. Solid State Chem. 223, 7 (2015).
27. J. Cisterna, C. Araneda, P. Narea, A. Cárdenas, J. Llanos, I. Brito, Molecules, 23, 2634 (2018).
28. M. A. Spackman, P. G. Byrom, Chem. Phys. Letters 267, 215, (1997).
29. A. Boultif, D. Loüer, J. Appl. Cryst. 37, 724, (2004).
30. A. Le Bail, Powder Diffr. 20, 316, (2005)
31. J. Rodríguez-Carvajal, Fullprof version 7.30, Laboratoire Léon Brillouin (CEA-CNRS), France, 2020.
32. Bruker, SMART and SAINT. Bruker AXS Inc. Madison, Wisconsin, USA, (1998).
33. G. M. Sheldrick, SADABS. University of Göttingen, Germany (1996).
34. G. M. Sheldrick, Acta Cryst. A. 64, 112, (2008).
35. O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, H. Puschmann H., J. Appl. Cryst. 42, 339 (2009).
36. G. M. Sheldrick, Acta Cryst. C, 71, 3 (2015).
37. C. R. Groom, I. J. Bruno, M. P. Lightfoot, S. C. Ward, Acta Cryst. B, 72, 171 (2016).
38. K. Brandenburg, DIAMOND, version 2.1e, Crystal Impact GbR, Bonn, Germany (2001).
39. A. L. Spek, Acta Cryst. D, 65, 148 (2009).
40. M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, P. R. Spackman, D. Jayatilaka, M. A. Spackman, CrystalExplorer17.5. University of Western Australia (2017).
41. M. A. Spackman, J. J. McKinnon. CrystEngComm, 4, 378 (2002).
42. F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen, R. Taylor, J. Chem. Soc. Perkin Trans. 2, S1 (1987).
43. M. C. Etter, J. C. MacDonald, J. Bernstein, Acta Cryst. B, 72, 256 (1990).