- Mechanical activation,
- Bentonite,
- α-FeOOH,
- Fenton-like,
- Catalytic activity
Copyright (c) 2017 Guangtao Wei, Zhongmin Li, Linye Zhang, Yue Deng, Luhua Shao, Zihan Liu

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
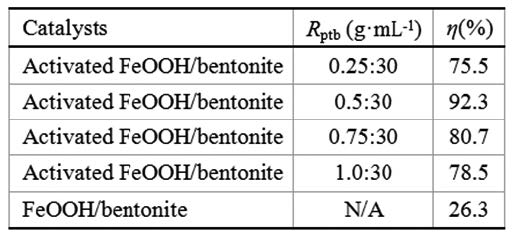
To improve the catalytic activity of FeOOH/bentonite material used in Fenton-like process, the activation of FeOOH/bentonite by mechanical activation was studied. The optimum conditions for activation of FeOOH/bentonite were as follows: filling ratio of grinding medium 30%, “15 mL of D06 balls plus 15 mL of D10 balls” as combination mode of grinding medium, rotation speed of planet carrier 600 rpm, milling time 40 min, and powder-to-ball ratio 0.5:30 (g·mL-1). The mechanical activation was an effective method to improve the catalytic activity of FeOOH/bentonite. Both the lattice distortion and crystal size decrease happened in α-FeOOH and the change of layer structure of bentonite were contributed to the increase of catalytic activity of activated FeOOH/bentonite.
References
- C. Feng, H.H. Sun, S.Q. Li, M.K. Camarillo, W.T. Stringfellow, Y.Y. Liang, Water Sci. Technol. 71, 1884–1892, (2015).
- M.D. Richmond, Water Environ. Res. 87, 650–655, (2015).
- L.Z. Zhang, H.H. Zeng, Y.M. Zeng, Z.H. Zhang, X.F. Zhao, J. Mol. Catal. A: Chem. 392, 202–207, (2014).
- A. Babuponnusami, K. Muthukumar, J. Environ. Chem. Eng. 2(1), 557– 572, (2014).
- S.H. Hu, Y.G. Wu, H.R. Yao, C. Lu, C.J. Zhang, Water Sci. Technol. 73(1), 153–160, (2016).
- A. Cihanoğlu, G. Gündüz, M. Dükkancı, Appl. Catal. B: Environ. 165, 687–699, (2015).
- L.H. Shao, G.T. Wei, Y.Z. Wang, Z.M. Li, L.Y. Zhang, S.K. Zhao, M. Zhou, Environ. Sci. Pollut. R. (2016), DOI:10.1007/s11356-016-6691-4.
- G.T. Wei, C.Y. Fan, L.Y. Zhang, R.C. Ye, T.Y. Wei, Z.F. Tong, Catal. Commun. 17, 184–188, (2012).
- S.T. Khankhasaeva, D.V. Dambueva, E.T. Dashinamzhilova, A. Gil, M.A. Vicente, M.N. Timofeeva, J. Hazard. Mater. 293, 21–29, (2015).
- L.Y. Zhang, S.Y. Cai, J.H. Mo, G.T. Wei, Z.M. Li, R.C. Ye, X.M. Xie, Mater. Manuf. Process. 30(3), 279–284, (2015).
- Q.Q. Chen, P.X. Wu, Y.Y. Li, N.W. Zhu, Z. Dang, J. Hazard. Mater. 168(2- 3), 901–908, (2009).
- L.Y. Zhang, S.Y. Cai, K. Huang, Z.M. Li, G.T. Wei, X.H. Li, J.H. Mo, Y.C. Liang, Desalin. Water Treat. 51(40-42), 7815–7824, (2013).
- J.X. Chen, L.Z. Zhu, Sep. Purif. Technol. 67(3), 282–288, (2009).
- M.A. Khaghani-Dehaghani, R. Ebrahimi-Kahrizsangi, N. Setoudeh, B. Nasiri-Tabrizi, Int. J. Refract. Met. H. 29(2), 244–249, (2011).
- K. G. Prashanth, Mater. Manuf. Process. 25(9), 974–977, (2010).
- A. Terzića, L. Pezo, L. Andrić, M. Arsenović, Compos Part B: Eng. 79, 660–666, (2015).
- S.F. Tikhov, V.A. Sadykov, K.R. Valeev, A.N. Salanov, S.V. Cherepanova, Y.N. Bespalko, V.E. Ramanenkau, Y.Y. Piatsiushyk, S.V. Dimov, Cataly. Today 246, 232–238, (2015).
- J. Choi, Y. Han, D. Kim, S. Park, J. Park, J. Park, H. Kim, Mater. Trans. 55(12), 1895–1899, (2014).
- M. Razavi, R. Ghaderi, M.R. Rahimipour, M.O. Shabni, Mater. Manuf. Process. 27(12), 1310–1314, (2012).
- A. Allahverdi, M. Mahinroosta, Powder Technol. 245(8), 182–188, (2013).
- V. Shojaei, M. Schaffie, A. Mohebbi, M. Ranjbar, Mater. Manuf. Process. 29(10), 1284–1288, (2014).


