Ag/CuO/MCM-48 AS A POTENTIAL CATALYST FOR THE SYNTHESIS OF SYMMETRICAL AND UNSYMMETRICAL POLYHYDROQUINOLINES

- Ag/CuO/MCM-48,
- Multicomponents reaction,
- One-pot synthesis,
- Polyhydroquinoline
Copyright (c) 2021 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
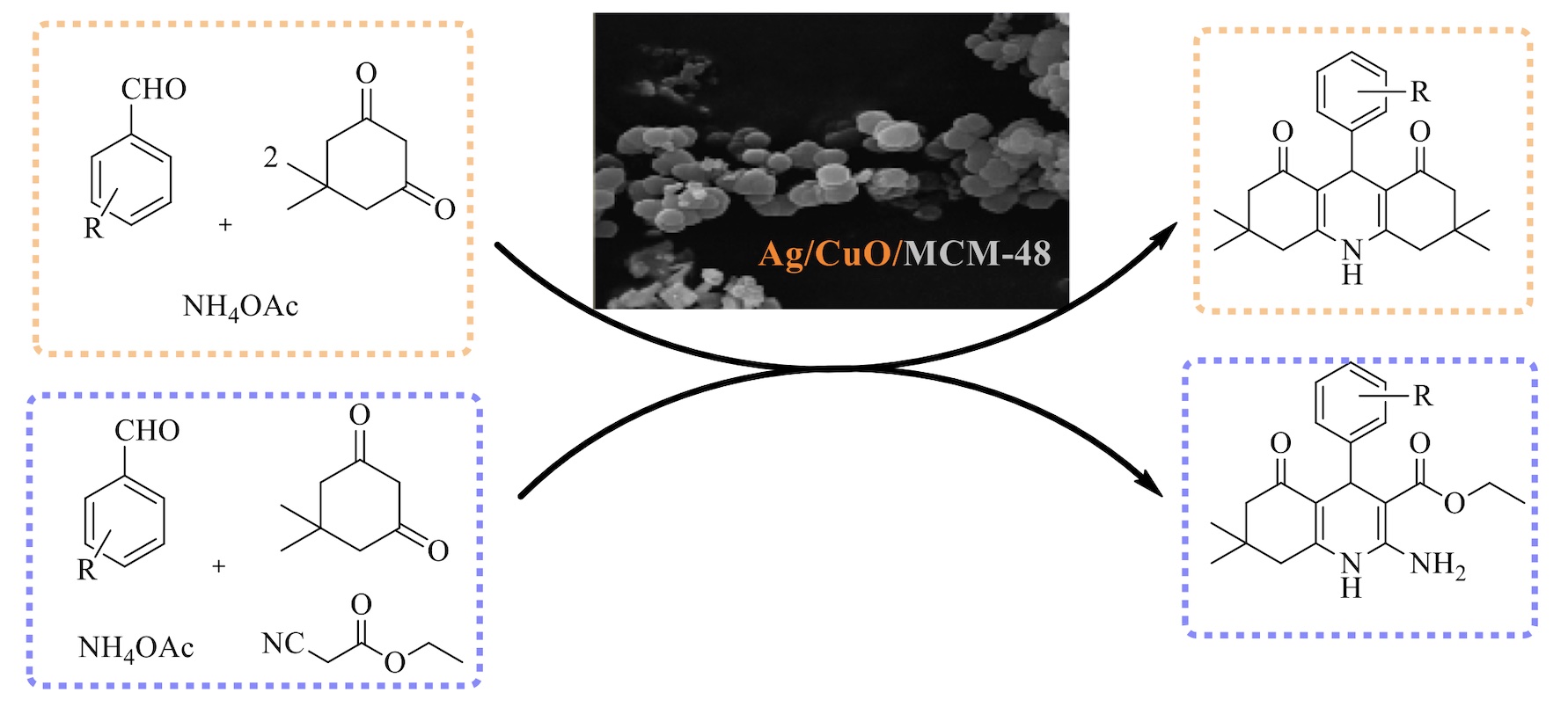
Ag/CuO/MCM-48 as a heterogeneous catalyst was efficiently employed in the synthesis of diversely substituted symmetrical and unsymmetrical polyhydroquinoline by a multi-component reaction of arylaldehyde, dimedone, ethyl cyanoacetate and ammonium acetate. This novel method is simple, environmentally friendly, rapid, use a recyclable catalyst and produce the products in high to excellent yields (83-97%) and lower reaction times (17-35 min). The catalyst can be reused at least 10 times without any appreciable decrease in its catalytic activities.
References
- - F. Bossert, H. Meyer, E. Wehinger, Angew. Chem., Int. Ed. Engl. 20, 762, (1981)
- - H. Nakayama, Y. Kasoaka, Heterocycles 42, 901, (1996)
- - T. Godfraid, R. Miller , M. Wibo, Pharmocol. Rev. 38, 321, (1986)
- - R. G. Bretzel, C. C. Bollen, E. Maester, K. F. Federlin, Drugs Future 17, 465, (1992)
- - P. N. Kalaria, S. P. Satasia, D. K. Raval, Eur. J. Med. Chem. 78, 207, (2014)
- - G. C. Rovnyak, S. D. Kimball, B. Beyer, G. Cucinotta, J. D. Dimarco, J. Gougoutas, A. Hedberg, M. Malley, J. P. MaCarthy, R. Zhang , S. Mereland, J. Med. Chem. 38, 119, (1995)
- - C. O. Kappe, W. M. F. Fabian, M. A. Semones, Tetrahedron 53, 2803, (1997)
- - K. Aouam , A. Berdeaux, Therapie 58, 333, (2003)
- - A. Hilgeroth, Mini-Rev. Med. Chem. 2, 235, (2002)
- - U. Eisner, J. Kuthan, Chem. Rev. 72, 1, (1972)
- - D. M. Stout, A. I. Meyers, Chem. Rev. 82, 223, (1982)
- - A. Sausins, G. Duburs, Heterocycles 27, 269, (1988)
- - N. Koukabi, E. Kolvari, A. Khazaei, M. A. Zolfigol, B. S. Shaghasemi, H. R. Khavasi, Chem. Commun. 47, 9230, (2011)
- - R. Ananthakrishnan, S.Gazi, Catal. Sci. Technol. 2, 1463, (2012)
- - L. Shen, S. Cao, J. Wu, J. Zhang, H. Li, N. Liu, X. Qian, Green Chem. 11, 1414, (2009)
- - M. M. Aghayan, R. Boukherroub, M. Nemati, M. Rahimifard, Tetrahedron Lett. 53, 2473, (2012)
- - A. Debache, R. Boulcina, A. Belfaitah, S. Rhouti, B.Carboni, Synlett 4, 509, (2008)
- - S. Thakrar, A. Bavishi, D. Bhavsar, S. Parekh, H. Vala, A. Radadiya, M. Parmar, M. Savant, Synth. Commun. 42, 3269, (2012)
- - S. Rostamnia, K. Lamei, Chin. Chem. Lett. 23, 930, (2012)
- - A. Kuraitheerthakumaran, S. Pazhamalaian, M. Gopalakrishnan, Chin. Chem. Lett. 22, 1199 (2011)
- - N. Koukabi, E. Kolvari, M. A. Zolfigol, A. Khazaei, B. S. Shaghasemi, B. Fasahati, Adv. Synth. Catal., 354, 2001, (2012)
- - R. Kumar, N. H. Andhare, A. Shard, A. Richa, K. Sinha. RSC. Adv. 4, 19111, (2014)
- - D. Langle, V. Marquardt, E. Heider, B. Vigante, G. Duburs, I. Luntena, D. Flotgen, C. Golz, C. Strohmann, Oliver Koch, D. Schade, Eur. J. Med. Chem. 95, 249, (2015)
- - F. Zaera, Chem. Soc. Rev. 42, 2746, (2013)
- - N. RaveendranShijuVadim V.Guliants, Appl. Catal., A 356,1, (2009)
- - A. H. Lu, E. L. Salabas, F. Schuth, Angew. Chem. Int. Ed. 46, 1222, (2007)
- - X. Wu, Y. Guo, L. Wan, C. Hu, J. Phys. Chem. 112, 16824, (2008)
- - A. G. Nasibulin, S. Rackauskas, H. Jiang, Nanopart. Res. 2, 373, (2009)
- - Z. Guo, D. Zhang, S. Wei, Z. Wang, B. Karki, J. Nanopart. Res. 12, 2415, (2010)
- -S. B. Sapkal,K. F. Shelke, B. B. Shingate, M. S. Shingare, Tetrahedron Lett. 50, 1754, (2009)
- - M. Abdollahi-Alibeik, S-S. Hoseinikhah, J. Iran Chem. Soc. 13, 1339, (2016)
- - R. J. Kalbasi, N. Mosaddegh, Catal. Commun. 12, 1231, (2011)
- - E. Vessally, R. Hosseinzadeh‐Khanmiri, E. Ghorbani‐Kalhor, M. Eshaghi, L. Ejlali, Appl. Organomet. Chem. 31, e3729, (2017)
- - W. Zhan, Y. Guo, Y. Wang, Y. Guo, X. Liu, Y. Wang, Z. Zhang, G. Lu, J. Phys. Chem. C 113, 7181, (2009)
- - N. Mosaddegh, I. Yavari, Chem. Pap. 72, 2013, (2018)
- - Y. Duan, D. Zhai, X. Zhang, J. Zheng, C. Li, Catal. Lett. 148, 51, (2018)
- - L. Kheirkhah, M. Mamaghani, A. Yahyazadeh, N. O. Mahmoodi, Appl. Organomet. Chem. 32, e4072, (2018)
- - M. Ahmadiazar, M. Mamaghani, Curr. Org. Chem. 22, 1326, (2018)
- - F. Tavakoli, M. Mamaghani, M. Sheykhan, N. Mohammadipour, M. Rassa, Curr. Org. Synth. 15, 872, (2018)
- - M. Mamaghani, M. Sheykhan, M. Sadeghpour, F. Tavakoli, Monatsh. Chem. 149, 1437, (2018)
- - P. Jahanshahi, M. Mamaghani, F. Haghbin, R. Hossein Nia, M. Rassa, J. Mol. Struct. 1155, 520, (2018)
- - F. Tavakoli, M. Mamaghani, M. Sheykhan, Appl. Organomet. Chem. 33, e5083, (2019)
- - P. Jahanshahi, M. Mamaghani, New J. Chem. 43, 8266, (2019)
- - F. Ramezanzadeh, M. Mamaghani, H. Fallah-Bagher Shaidaei, M. Sheykhan, Polycycl. Aromat. Comp. DOI: 10.1080/10406638.2019.1705360, (2019)
- - E. Saberikhah, M. Mamaghani, N. O. Mahmoodi,; A. Fallah Shojaei, Polycycl. Aromat. Comp. DOI: 10.1080/10406638.2020.1729821, (2020)
- - A. Kumar, S. Sharma, V. D. Tripathi, R. A. Maurya, S. P. Srivastava, G. Bhatia, A. K. Tamrakar, A. K. Srivastava, Bioorg. Med. Chem. 18, 4138, (2010)
- - C. Witpathomwong, R. Longloilert, S. Wongkasemjit, S. Jitkarnka, Energy Procedia 9, 245, (2011)
- - G. M.Ziarani, A. Badiei, M. Hassanzadeh, S. Mousavi, Arab. J. Chem. 7, 335, (2014)
- - G.-W. Wang, J.-J.Xia, C.-B. Miao, X.-L. Wu, Bull. Chem. Soc. Jpn. 79, 454, (2006)
- - S. Hashemi-Uderji, M. Abdollahi-Alibeik, R. Ranjbar-Karimi, Main Group Met. Chem. 41, 91, (2018)
- - K. Ramesh, M. Pasha, Bioorg. Med. Chem. Lett. 24, 3907, (2014)
- - M. Saha, A. K. Pal, Tetrahedron Lett. 52, 4872, (2011)
- - M. Abdollahi-Alibeik, A. Rezaeipoor-Anari, J. Magn. Magn. Mater. 398, 205, (2016)

