SYNTHESIS, CRYSTAL STRUCTURE AND PHOTOLUMINESCENCE PROPERTIES OF A NEW RARE-EARTH CARBONATE Na3Eu(CO3)3·6H2O
- Hydrothermal,
- Carbonate,
- X-ray diffraction,
- Photoluminescence
Copyright (c) 2017 Rong-Hua Zhang, Dan Zhao, Min Huang, Rui-Juan Yang, Fa-Xue Ma, Yun-Chang Fan

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
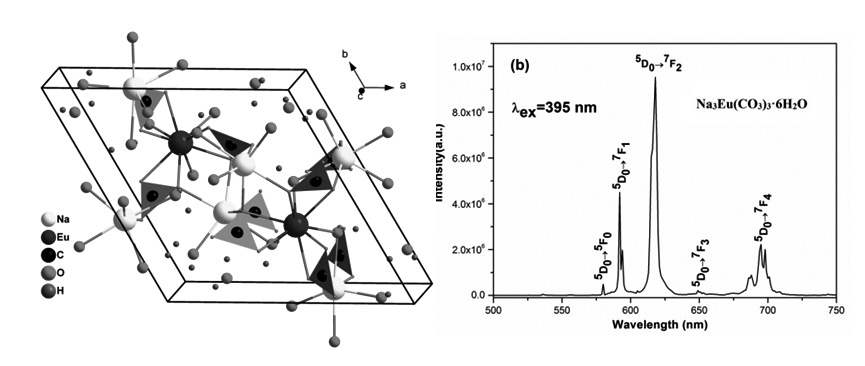
A new sodium europium carbonate hydrate, Na3Eu(CO3)3·6H2O, has been prepared using the hydrothermal method, and its structure was determined by single crystal X-Ray diffraction analysis for the first time. It crystallizes in the polar hexagonal space group P63 with a = 11.382(9) Å, c = 5.989(5) Å, V = 672.06(9) Å3, Z = 2, Mr = 509.06, Dc = 2.516 g/cm3, F(000) = 492, μ(MoKα) = 4.84 mm-1, R1 = 0.015 and wR = 0.038. The structure features a three-dimensional(3D) framework composed of planar (CO3)2- triangles, Eu3+ ions and Na+ ions, which delimits 1D infinite chains with a hexangular star appearance along the c-axis. The IR spectrum and the self-activated photoluminescence properties were studied. Under the excitation of near UV light (395nm), it shows strong red emission at 618 nm with a lifetime of 348.7 μs. Present research indicates that Na3Eu(CO3)3·6H2O is a promising red phosphor for white light-emitting diodes.
References
- D. Wawrzynczyk, M. Samoc, Cryst. Eng. Comm. 17, 1997 (2015).
- W. Xiao, X. Zhang, Z. Hao, G. H. Pan, Y. Luo, L. Zhang, J. Zhang, Inorg. Chem. 54, 318, (2015).
- X. Xu, W. Dong, J. Ding, Y. Feng, F. Zhang, L. Ma, Z. J.Peng,Fabrication of Y3Al5O12:Eu thin films and powders for field emission display applications J. Nanosci. Nanotechnol. 15, 543, (2015).
- M. Luo, Y. Song, C. Lin, N. Ye, W. Cheng, X. F. Long, Chem. Mater. 28, 2301, (2016).
- K. V. Raju, C. N. Raju, S. Sailaja, S. J. Dhoble, B. S. Reddy, J. Lumin. 134, 297, (2013).
- X. Li, L. Guan, M. S. Sun, H. Y. Liu, Z. p. Yang, Q. L. Guo, G. S. Fu, J. Lumin. 131, 1022, (2011).
- D. Rajesh, M. Dhamodhara Naidu, Y. C. Ratnakaram, J. Phys. Chem. Solids. 75, 1210, (2014).
- S. Hachani, B. Moine, A. El-akrmi, M. Férid, Opt. Mater. 31, 678, (2009).
- N. Ye, C. Tu, X. Long, X. Long, Cryst. Growth Des. 10 , 4672, (2010).
- U. Rambabu, S. Buddhudu, Opt. Mater. 17, 401, (2009).
- M. Luo, N. Ye, G. Zou, C. Lin, W. Cheng, Chem. Mater. 25, 3147, (2009).
- E. Antic-Fidancev, M. Lemaitre-Blaisd, P. Porcher, N. Mercier, M. Leblanc, J. Solid State Chem. 116, 286, (1995).Optical Properties of BaEu(CO3)2 F and Na3La2(CO3)4F: Eu3+: Correlations to the Crystallographic Structures.
- N. Mercier, M. Leblanc, F. Antic-Fidaancev, M. Lemaitre-Blaise, P. Porcher, J. Solid State Chem. 132, 33, (1995).
- N. Mercier, N. Leblanc, E. Antic-Fidancev, M. Lemaitre-Blaise, P. Porcher, J. Alloys Comp. 225, 198, (1995).
- G. B. Stringfellow, M. G. Craford, Semiconduct. Semimet. 48, 1, (1997).
- A. Karmakar, I. Goldberg, Cryst. Eng. Comm. 13, 339, (2011).
- T. Beck, A. Krasauskas, T. Gruene, G. M. Sheldrick, Acta Crystallogr. 64, 1179, (2008).
- G. M. Sheldrick, Acta Crystallogr. A. 4, 12, (2008).
- J. D. Grice, Can. Mineral. 34, 649, (1996).
- I. V. Pekov, N. V. Chukanov, N. V. Zubkova, D. A. Ksenofontov, L. Horvath, A. E. Zadov, D. Y. Pushcharovsky, Can. Mineral. 48, 95, (2010).
- J. D. Grice, J.V. Velthuizen, R. A. Gault, Can. Mineral. 34, 649, (1996).
- L. Shen, L. Xiao, J. Phys. D: Appl. Phys. 45, 115, (2012).
- M. Luo, G. Wang, C. Lin, N. Ye, Y. Zhou, W. Cheng, Inorg. Chem. 53, 80, (2014).
- A. Jouini, M. Fe´rid, M. Trabelsi-Ayad, Thermochim Acta. 400, 199, (2003).
- J. Chen, M. Luo, N. Ye, Z. Anorg. Allg. Chem. 641, 460, (2015).
- J. Niu, J. Zhao, J. Wang, Y. Bo, J. Coord. Chem. 57, 935, (2004).
- G. Zou, N. Ye, L. Huang, X. Lin, J. Am. Chem. Soc. 133, 20001, (2011).
- C. C. Lin, R. S. Liu, J. Phys. Chem. Lett. 2, 1268, (2011).
- Q. Su, H. B. Liang, Li, Y. C. H. He, Y. Lu, H.; J. Li, Y. Tao, J. Lumin. 122, 927, (2007).
- J. Wang, J. Zhao, J. Niu, Sci. China Ser. B. 48, 343, (2005).
- G. B. Stringfellow, M. G. Craford, Academic Press. 6, 247, (1997).


