Synthesis, Chemical Identification, Drug Release and Docking Studies of the Amlodipine–Chitosan Nanobiopolymer Composite
- Amlodipine,
- Chitosan,
- Nanocomposite,
- Drug-release properties,
- Docking studies
Copyright (c) 2021 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
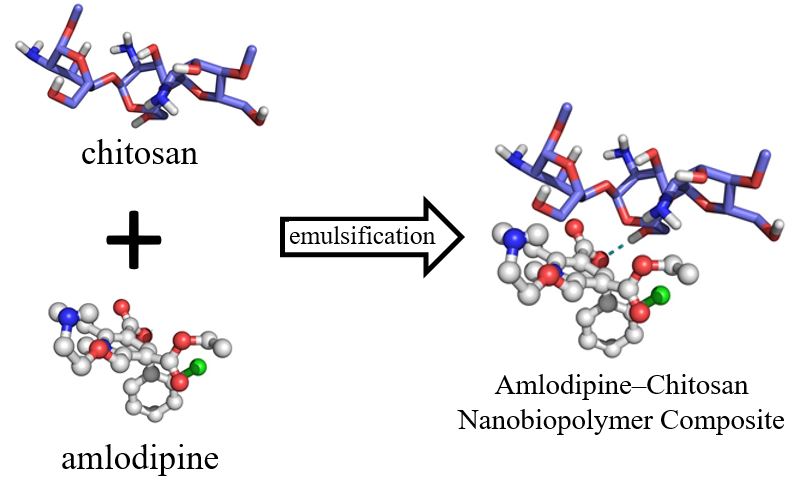
A new amlodipine-chitosan nanocomposite was built using amlodipine nanoparticles as primary scaffolds by spontaneous emulsification, and its complete elucidation was performed by using several spectrometric techniques. Our results indicate that the amlodipine-chitosan nanocomposite has better solubility than amlodipine at pH 7.4 with a nearly all the drug substance dissolved (97%) by the final time-point measured. The docking study support the existence of intermolecular interactions are established between amlodipine and chitosan.
References
2. S.N. Kokil, PhD Dissertation, Long Island University, THE BROOKLYN CENTER, 2009, 215 pages; 3371316 (2009). http://search.proquest.com/docview/304994657
3. D.M. Brahmankar, and S.B. Jaiswal, 1st ed. Delhi: Vallabh Prakashan, (2006), 296.
4. S.G. Kapsi, and J.W. Ayres, Int J Pharm. (2001), 23(229), 193-203.
5. Xi. Han, C. Ghoroi, D. To, Y. Chen, and R. Davé, Int J Pharm. (2011), 415, 185-195.
6. G.G. Liversidge, and K.C. Cundy, Int J Pharm. (1995), 125, 91-97.
7. R.H. Müller, K. Peters, Int J Pharm. (1998), 160, 229-237.
8. S. Ghanbarzadeh, H. Valizadeh, and P. Zakeri-Milani, Adv Pharm Bull. (2013), 3, 25-29.
9. S.F. Wang, F. Gu, and M.K. Lu, Langmuir (2006), 22: 398.
10. J.Z. Xu, S. Xu, J. Geng, G.X. Li, and J.J. Zhu, Ultrason Sonochem. (2006), 13, 451-454.
11. S.Y. Zhang, Y. Liu, X. Ma, and H.Y. Chen, J Phys Chem B. (2006), 110(18), 9041-9047.
12. H. Lei, Y.J. Tang, J.J. Wei, J. Li, X.B. Li, and H.L. Shi, Ultrason Sonochem. (2007), 14(1): 81-83.
13. V.G. Kumar, and K.B. Kim, Ultrason Sonochem. (2006), 13(6): 549-556.
14. C.J. Mao, H.C. Pan, X.C. Wu, J.J. Zhu, and H.Y. Chen, J Phys Chem B. (2006), 110(30), 14709-14713.
15. A. Lashgari, S. Ghamami, S. Shahbazkhany, G. Salgado-Morán, and D. Glossman-Mitnik, J Nanomater. Article ID 384835 (2015). Doi:10.1155/2015/384835.
16. V.G. Dongre, S.B. Shah, P.P. Karmuse, M. Phadke, and V.K. Jadhav, J Pharm Biomed Anal. (2008), 46, 583e586.
17. A.K. Sarkar, D. Ghosh, A. Das, P.S. Selvan, K.V. Gowda, U. Mandal, A. Bose, S. Agarwal, U. Bhaumik, and T.K. Pal, .J Chromatogr B. (2008), 873(1):77–85.
18. WHO Expert Committee on Specifications for Pharmaceutical Preparations - WHO Technical Report Series, No. 992 - Forty-ninth Report (Geneva, 13–17 October 2014)
19. T.M. Allen, and P.R. Cullis, Science (2004), 303: 1818–1822.
20. I. Aranaz, M. Mengíbar, R. Harris, I. Panos, B. Miralles, N. Acosta, G. Galed, and A. Heras, Curr Chem Biol. (2009), 3(2):203–230.
21. Y. Shi, A. Wan, Y. Shi, Y. Zhang, and Y. Chen, Biomed Res Int. (2014), Article ID 613619. doi: 10.1155/2014/613619
22. O. Felt, P. Buri, and R. Gurny, Drug Dev Ind Pharm. (1998), 24:979–993.
23. A. Di Martino, M. Sittinger, and M.V. Risbud, Biomaterials, (2005), 26:5983–5990.
24. W.E. Rudzinski, and T.M. Aminabhavi, Int J Pharm. (2010), 399:1–11.
25. S.A. Agnihotri, and T.M. Aminabhavi, Drug Dev Ind Pharm. (2007), 33:1254–1262.
26. S.C. Angadi, L.S. Manjeshwar, and R.M. Aminabhavi, Ind Eng Chem Res. (2013), 52: 6399–6409.
27. G. Chhabra, K. Chuttani, A.K. Mishra, K. Pathak, Drug Dev Ind Pharm. (2011), 37:907–916.
28. ACD/Structure Elucidator, version 15.01 [Internet]. Toronto (ON): Advanced Chemistry Development, Inc. c1996-2015- www.acdlabs.com
29. M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, and et al Gaussian 98 Revision A.7. Gaussian Inc, Pittsburgh, P.A, 1998.
30. G.M. Morris, R. Huey, W. Lindstrom, M.F. Sanner, R.K. Belew, D.S. Goodsell, and et al. J. Comput. Chem. (2009), 30:2785-2791.
31. L.L.C. Schrödinger, The PyMOL Molecular Graphics System [type of medium]. Version 1.7.4. 2019
32. V. Koradia, H.L. de Diego, K. Frydenvang, M. Ringkjøbing-Elema, A. Mullertz, A.D. Bond, and et al. Cryst Growth Des., (2010), 10(12):5279-5290.
33. V. Koradia, A.F. de Lemos, M. Allesø, H.L. d e Diego, M.R. Ringkjøbing-Elema, A. Mullertz, and et al., J Pharm Sci. (2011), 100:2896-2910.
34. A. Lashgari, S. Ghamami, Z.Bahrami, F. Shomossi, G. Salgado-Morán, and D. Glossman-Mitnik, J Nanomater., Article ID 130698, 2015. doi:10.1155/2015/130698.
35. S. Ghamami, M. Golzani, and A. Lashgari, J. Chil. Chem. Soc. (2016), 61(2):2954-2957.
36. A. Lashgari, S. Ghammamy, L. Gerli, and G. Salgado Morán, Advances in Environmental Biology, (2014), 8(21):339-345.
37. S. Ghamami, S. Kazemzade Anari, M. Bakhshi, A. Lashgari, G. Salgado-Morán, and D. Glossman-Mitnik, Open Chem. (2016), 14, 60–64.
38. A. Lashgari, S. Ghammami, M. Golzani, L. Gerli-Candia, G. Salgado-Morán, D. Glossman-Mitnik, and B. Abdolmaleki, J. Chil. Chem. Soc. (2016), 61(4), 3201-3205.
39. L. Szabó, V. Chiş, A. Pîrnău, Leopold N., Cozar O., and Orosz S. J. Mol. Struct., (2009), 924-926: 385-392.
40. Y. Shao, L. Li, X. Gu, L. Wang, and S. Mao, Asian J Pharm Sci. (2015), 10:24–30.
41 E. Ramírez-San Juan, M.A. Soriano-Ursúa, J.E. Raya, J. Correa-Basurto, J.G. Trujillo-Ferrara, R. Miranda-Ruvalcaba, and et al., Med Chem Res. (2014), 32(12): 5149-5159.
42. S. Ghamami, M. Golzani, and A. Lashgari, J Incl Phenom Macrocycl Chem. (2016), 86(1): 67-78.
43. S. Ghamami, A. Lashgari, and M. Golzani, J Incl Phenom Macrocycl Chem. (2017), 88(1):1-14.