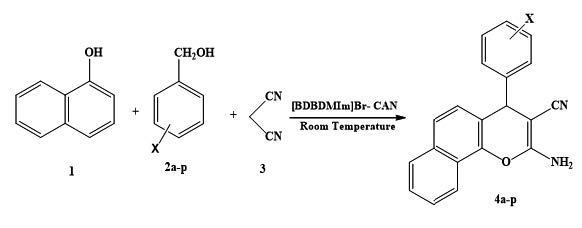
GRINDING SYNTHESIS OF 2-AMINO-4H-CHROMENES USING 3,3-(BUTANE-1,4-DIYL) BIS (1,2-DIMETHYL- 1H-IMIDAZOLE-3-IUM)Br-CAN AS A NOVEL REAGENT
- Chromenes,
- Benzyl alcohols,
- Ionic Liquid,
- Multicomponent reaction,
- 1-Naphthol
- Malononitrile ...More
Copyright (c) 2017 Mohammad Nikpassand, Leila Zare Fekri, Parvin Ahmadi

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
A clean and environmentally benign route to 2-amino-4H-chromenes has been developed via three-component condensation reaction of various benzyl alcohols, malononitrile and 1-naphthol, using a catalytical amount of CAN and a reusable ionic liquid 3,3-(Butane-1,4-diyl)bis(1,2-dimethyl-1H-imidazole-3-ium) bromide ([BDBDMIm]Br) as a catalyst at room temperature. The present methodology offers several advantages such as solvent-free conditions, excellent yields, simple procedure, mild conditions and reduced environmental consequences. The ionic liquid was recovered and reused. All of synthesized compounds were characterized by IR, NMR and elemental analyses.
References
- Z. Bard, W. Y. Dua, J. Siau, J. Wang, Tetrahedron. Lett. 52, 6137, (2010).
- L. Moafi. S. Ahadi, A. Bazgir, Tetrahedron. Lett. 51, 6270, (2010).
- D. J. Maloney, S. M. Hecht, Org. Lett. 7, 4297, (2005).
- M. Curini, G. Cravotto, F. Epifano, G. Giannone, Curr. Med. Chem. 13, 199, (2006).
- R. O. Kennedy, R. D. Thornes, Suggested Modes of Action of Coumarins and Some Comments on their Significance. Coumarins: Biology, Applications and Mode of Action, John Wiley & Sons: Chichester. (1997).
- G. P. Ellis, In the Chemistry of Heterocyclic Compounds. Chromenes, Chromanes, and Chromones; Weissberger A, Taylor E C Eds, John Wiley: New York, 11, (1977).
- W. P. Smith, L. S. Sollis, D. P. Howes, C. P. Cherry, D. I. Starkey, N. K. Cobley, J. Med. Chem. 41, 787, (1998).
- G. A. Kraus, I. Kim, J. Org. Chem. 68, 4517, (2003).
- K. Hiramoto, A. Nasuhara, K. Michiloshi, T. Kato, K. Kikugawa, Mutat. Res. 395, 47, (1997).
- J. G. Tangmouo, A. L. Meli, J. Komguem, V. Kuete, F. N. Ngounou, D. Lontsi, V. P. Beng, M. I. Choudhary, B. L. Sondengam, Tetrahedron. Lett. 47, 3067, (2006).
- R. O. S. Kitamura, P. Romoff, M. C. M. Young, M. J. Kato, J. H. G. Lago, Phytochemistry. 67, 2398, (2006).
- S. Makarem, A. A. Mohammadi, A. R. Fakhari, Tetrahedron Lett. 49, 7194, (2008).
- Zh. Zhou, F. Yang, L. Wu, A. Zhan, Chem. Sci. Trans. 1, 57, (2012).
- S. Khaksar, A. Rouhollahpour, S. Mohammadzadeh Talesh, J. Fluorine. Chem. 141, 11, (2012).
- S. R. Kale, S. S. Kahandal, A. S. Burange, M. B. Gawande, R. V. Jayaram, Catal. Sci. Technol. 3, 2050, (2013).
- K. Gong, H. L. Wang, J. Luo, Z. L. Liu, J. Hetero. Chem. 46, 1145, (2009).
- S. K. Kundu, J. Mondal, A. Bhaumik, Dalton. Trans. 42, 10515, (2013).
- T. Welton, Chem. Rev. 99, 2071, (1999).
- R. Sheldon, Chem. Commun. 2399, (2001).
- J. K. Lee, M. J. Kim, J. Org. Chem. 67, 6845, (2002).
- M. Nikpassand, L. Zare Fekri, P. Farokhian, Syn. Commun. 45, 2303, (2015).
- M. Nikpassand, M. Mamaghani, F. Shirini, K. Tabatabaeian, Ultrason. Sonochem. 17, 301, (2010).
- M. Nikpassand, L. Zare, M. Saberi, Monatsh. Chem. 143, 289, (2012).
- L. Zare Fekri, M. Nikpassand, K. Hassanpour, Curr. Org. Chem. 12, 76, (2015).
- Nair, V. Deepthi, A. Chem. Rev. 107, 1862, (2007).
- W. S. Trahanovsky, L. B. Young, G. L. Brown, J. Org. Chem. 32, 3865, (1967).


