PHENOLICS COMPOSITION, ANTIOXIDANT AND CORROSION INHIBITION EFFICIENCY, CAPACITY OF Fraxinus excelsior EXTRACTS.

- Fraxinus excelsior,
- ABTS,
- FRAP,
- DPPH,
- Antioxidant Capacity
- Corrosion inhibitor. ...More
Copyright (c) 2021 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
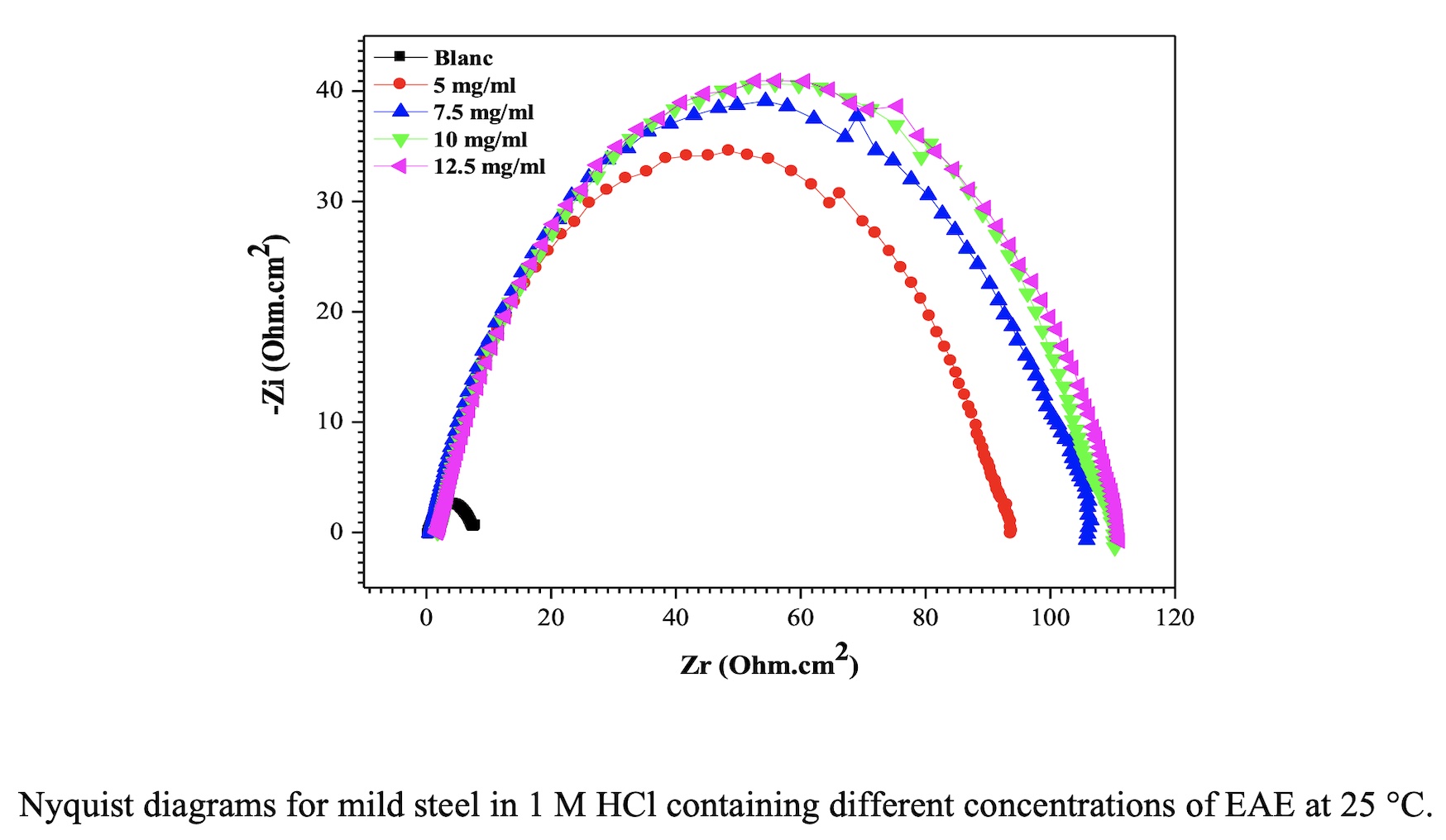
Objective: In this study, we will determine the antioxidant properties of of Fraxinus excelsior extracts and will correlate the values with total levels of polyphenolic compounds. And the ethyl acetate extract (EAE) was investigated as eco-friendly corrosion inhibitor of carbon steel in 1M HCl has been studied by both electrochemical impedance spectroscopy (EIS) and Tafel polarization measurements. Methods: Antioxidant activity was evaluated by DPPH, ABTS+, FRAP and enzymatic test. Total polyphenols and total flavonoids contents were evaluated by spectrophotometric assays. And the corrosion inhibition was studied by polarization and electrochemical impedance spectroscopy methods. Results: The total polyphenols and flavonoids contents were in the order EAE > ChE > BolE > MetE > AqE > PEE. The DPPH of MetE, PEE, EAE, ChE, BolE and AqE extract activity expressed as IC50 values were in the order 125.15 ± 2.5, 3722.5 ± 31.82, 23.1 ± 0.48, 101.86 ± 1.64, 106.06 ± 2.97 and 363.41±1.38 µg / ml. The results of ABTS.+, FRAP and the enzymatic test showed that the Fraxinus excelsior extracts have a potential antioxidant activity in the same order obtained by using the DPPH test, which the antioxidant activity followed a decreasing order: EAE > ChE > BolE > MetE > AqE > PEE. The ethyl acetate extract (EAE) exhibits good inhibition properties for the corrosion of carbon steel in 1M HCl solution. Conclusions: In conclusion, Fraxinus excelsior extracts contain active compounds which have antioxidant effects and can be useful in the treatment of pathologies where these activities are neede. And the corrosion inhibition data considered these extract (EAE) as efficient corrosion inhibitor.
References
- S. Alok, S.K. Jain, A. Verma, M. Kumar, A. Mahor, M. Sabharwal, Asian Pac. J. Trop. Biomed. 4, 78, (2014).
- M. Sgorbini, F. Bonelli, A. Rota, P. Marmorini, G. Biagi, M. Corazza. A. Pasquini, Theriogenology. 83, 48, (2015).
- T. Finkel, N. J. Holbook, Nature. 408, 239, (2000).
- S. Melov, J. Revenscroft, S. Malik, M. S. Gill, D. W. Walker, P. E. Clayton, Science. 289, 1567, (2000).
- O. Raouf, A. R. Patrice, B. Andre, W. Jean-Michel, T. Yvan, Life Sci. 68, 387, (2000).
- R. Scherer, H. T. Godoy, Food Chem. 112, 654, (2009).
- D. H Luo, B.S. Fang, Carbohydr. Polym. 72, 376, (2008).
- K. J. Anderson, S. S. Teuber, A. Gobeille, P. Cremin, A. L. Waterhouse, F. M. Steinberg, J. Nutr. 131, 2837, (2001).
- D. Marquardt, J. A. Williams, N. Kucerka, J. Atkinson, S. R. Wassall, J. Katsaras, T. A. Harroun, J. Am. Chem. Soc. 135, 7523, (2013).
- P. Visen, B. Saraswat, A. Visen, M. Roller, C. Mermet, K. He, N. Bai, B. Lemaire, S. Lafay, A. Ibarr, J. Ethnopharmacol. 126, 226, (2009).
- N. Bai, K. He, M. Rolle, C. S. Ching-Shu Lai, X. Shao, M. H. Pan, C. T. Ho, Am. Chem. Soc. 2, 115, (2013).
- A. El Bribri, M. Tabyaoui, B. Tabyaoui, H. El Attari, F. Bentiss, Mat. Chem. Phys. 141, 240, (2013).
- A. Singh, E.E. Ebenso, M. A. Quraishi, Int. J. Corros. 2012, 1, (2012).
- D. Benmessaoud Left, M. Zertoubi, A. Irhzo, M. Azzi, Mater. Environ. Sci. 4, 855, (2013).
- A. K. Satapathy, G. Gunasekaran., S.C. Sahoo, Kumar Amit, P.V. Rodrigues, Corros. Sci. 51, 2848, (2009).
- A. Minhaj, P. A. Saini, M. A. Quraishi, I. H. Farooqi, Corros. Prev. Control, 46, 32, (1999).
- K. R. Markham, Academic Press. Techniques of flavonoid identification, London 1982; p 133.
- S. Boumerfeg, A. Baghiani, D. Messaoudi, S. Khennouf, L. Arrar, Phytother. Res. 23, 283, (2009).
- T. Bahorun, B. Gressier, F. Trotin, C. Brunet, T. Dine, M. Luyckx, J. Vasseur, M. Cazin, J. C. Cazin, M. Pinkas, Arzneimittel-forschung. 46, 1086, (1996).
- S. Boumerfeg, A. Baghiani, M. Djarmouni, D. Ameni, M. Adjadj, F. Belkhiri , N. Charef, S. Khennouf, L. Arrar, Chinese Medi. 3, 30, (2012).
- A. Baghiani, D. Ameni, S. Boumerfeg, M. Adjadj, M. Djarmoun, N. Charef, S. Khenouf, L. Arrar, Am. J. Medici. Med. Sci., 2, 25, (2012).
- R. Samarth, M. Panwar, M. Kumar, A. Soni, Food chem. 106, 868, (2008).
- R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, Free rad. bio. Med. 26, 1231, (1999).
- I. F. Benzie, J. J. Strain, Anal. Bio. Chem. 239, 70, (1996).
- NCCLS (National Committee for Clinical Laboratory Standards) Performance Standards for Anti-Microbial Susceptibility Testing: Eleventh Informational Supplement. Wayne, PA, USA: National Committee for Clinical Laboratory Standard; 2001. NCCLS, Document M 100-S11.
- M. Tourabi, K. Nohair, M. Traisnel, J. Jama, F. Bentiss. Corros. Sci. 75, 123, (2013).
- H. Zarrok, A. Zarrouk, B. Hammouti, R. Salghi, C. Jama, F. Bentiss. Corros. Sci. 64, 243, (2012).
- M. P. Kahkonen, A. I. Hopia, H. J. Vuorela, J. P. Rauha, K. Pihlaja, T. S. Kujala, J. Agric. Food Chem. 47, 3954, (1999).
- C. A. Rice-Evans, N. J. Miller, P. G. Bolwell, P. M. Bramley, J. B. Pridham, Free Rad. Res. 22, 375, (1995).
- A. S. Boath, D. Grussu, D. Stewart, G. J. Mc Dougall, Food Dig. 3, 1, (2012).
- R. Zaidi-Yahiaoui, F. Zaidi, A. Ait Bessai, Afr. J. Biotech. 7, 282, (2008).
- M. Umamaheswari, A. Madeswaran, K. Asokkumar, T. Sivashanmugam, V. Subhadradevi, P. Jagannath, Der. Pharma. Chemica. 3, 240, (2011).
- P. Cos, L. Ying, M. Calomme, J. P. Hu, K. Cimanga, B. Van-Poel, L. Pieters, A. J. Vlietinck, D. Vanden-Berghe, J. Nat. Prod. 61, 71, (1998).
- P. Montoro, A. Braca, C. Pizza, N. De Tommasi, Food Chem. 92, 349, (2005).
- B. Halliwell, Lancet. 344, 721, (1994).
- Y. Z. Cai, Q. Luo, M. Sun, H. Corke, Life Sci. 74, 2157, (2004).
- J. S. Bao, Y. Cai, M. Sun, G. Y. Wang, H. Corke, J. Agric. Food Chem. 53, 2327, (2005).
- M. Hidalgo, C. Sánchez-Moreno, S. Pascual-Teresa, Food Chem. 121, 691, (2010).
- J. Taira, E. Tsuchida, M. C. Katoh, M. Uehara, T. Ogi, Food Chem. 166, 531, (2015).
- M. R. Saha, A. Alam, R. Akter, R. Juhangir, Bangladesh J. Pharmacol, 3, 90, (2008).
- R. Q. Ferreira, S. J. Greco, M. Delarmelina, K. C. Weber, Electrochim. Acta. 163, 161, (2015).
- T. Nagai, I. Reiji, I. Hachiro, S. Nobutaka, Food Chem. 80, 29, (2003).
- I. F. Benzie, J. J. Strain, Method. Enzymol. 299, 15, (1999).
- R. A. Moyer, K. E. Hummer, C. E. Finn, B. Frei, R. E. Wrolstad, J. Agric. Food Chem. 50, 519, (2002).
- A. Luximon-Ramma, T. Bahorun, M. A. Soobrattee, O. I. Aruoma, J. Agric. Food Chem. 50, 5042, (2002).
- R. Pulido, L. Bravo, F. Saura-Calixto, J. Agric. Food Chem. 48, 3396, (2000).
- S. H. Kumar, S. Karthikeyan, Ind. Eng. Chem. Res. 52, 7457, (2013).
- D. K. Yadav, M. A. Quraishi, Ind. Eng. Chem. Res. 51, 8194, (2012).
- F. S. de Souza, A. Spinelli, Corros. Sci. 51, 642, (2009).
- C. B. Pradeep Kumar, K. N. Mohana, J. Tai. Inst. Chem. Eng. 45, 1031, (2014).
- A. Ghames, T. Douadi, S.Issaadi, L. Sibous, K. I. Alaoui, M. Taleb, S. Chafaa, Int. J. Electrochem. Sci. 12, 4867, (2017).
- R. A. Rikkouh, T. Douadi, H. Hamani, M. Al-Noaimi, S. Chafaa, J. Adhes. Sci. Tech. 34, 1, (2020).
- H. Debab, T. Douadi, D. Daoud , S. Issaadi, Int. J. Electrochem. Sci. 13, 6958, (2018).
- M. Mahdavian, M. Attar, Corros. Sci. 51, 409, (2009).
- M. Scendo, J. Uznanska, Int. J. Corros. (2011). https://doi.org/10.1155/2011/718626.
- Y. Sasikumar, A.S. Adekunle, L.O. Olasunkanmi, I. Bahadur, R. Baskar, M.M. Kabanda, I.B. Obot, E.E. Ebenso, J. Mol. Liqu. 211, 105, (2015).
- A. K Singh, S. K. Shukla, M. A. Quraishi, E. E. Ebenso, J. Chin. Inst. Chem. Eng. 43, 463, (2012).
- A. S. Fouda, A. Eldesoky, A. El-Sonbati, S. Salam, Int. J. Electrochem. Sci. 9, 1867, (2014).
- A. S. Fouda, F. El-Taib Heakal, M. S. Radwan, J. Appl. Electrochem. 39, 391, (2009).
- J. Aljourani, K. Raeissi, M.A. Golozar, Corros. Sci. 51, 1836, (2009).
- O. Benali, L. Larabi, M. Traisnel, L. Gengenbre, Y. Harek, Appl. Surf. Sci. 253, 6130, (2007).
- K. C. Emregu, M. Hayvali, Corros. Sci. 48, 797, (2006).
- C. M. Goulart, A. Esteves-Souza, C. A. Martinez-Huitle, C. J. F. Rodrigues, M. A. M.
- Maciel, A. Echevarria, Corros. Sci. 67, 281, (2013).
- D. Daoud, T. Douadi, S. Issaadi, S. Chafaa, Corros. Sci. 79, 50, (2014).
- S. Muralidharan, N. Phani, S. Pitchumani, S. Ravichandran, S. Iyer Venkatakrishna, J. Electrochem. Soc. 142, 1478, (1995).

