ELECTRODE MODIFIED WITH A POLYMER OF ANILINE AND 3-HEXYLTHIOPHENE TO BE ASSAYED IN THE SELECTIVE DETERMINATION OF NITRATE

- conducting polymer,
- ion selective electrode,
- nitrate detection,
- nitrate sensor,
- polyaniline
- polythiophene ...More
Copyright (c) 2020 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
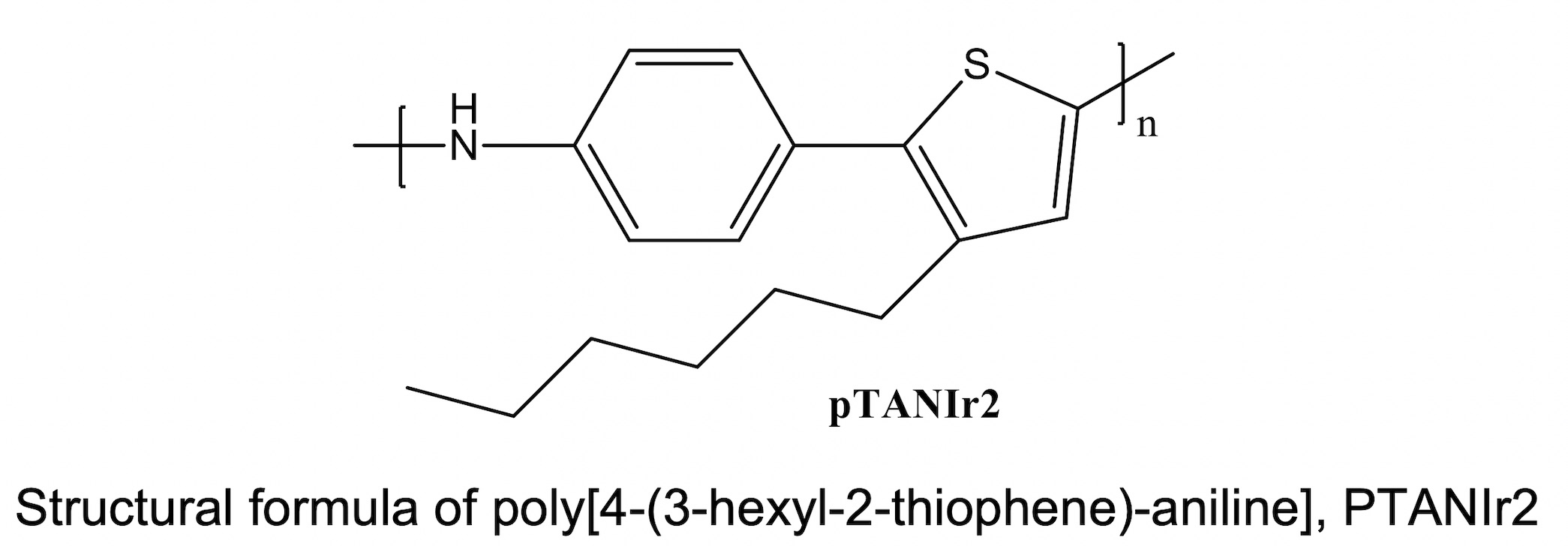
In this work, platinum electrodes are modified with poly[4-(3-hexyl-2-thiophene)-aniline], PTANIr2, and characterized by cyclic voltammetry. In the presence of nitrate in the electrolytic medium, an oxidation signal at 0.6 V vs. SCE is recorded, so the response of this modified electrode, Pt|PTANIr2, is evaluated to determine its relationship with the concentration of the anion. Thus, it is verified that the best response is achieved by chrono-amperometry, obtaining a directly proportional relationship between the charge and the concentration, in an interval between 5 and 100 mg L-1 of the anion, with r2 = 0.996. Furthermore, it is found that the response of Pt|PTANIr2 is quite selective for nitrate, since there is no response for anions such as HPO4-, PO42-, ClO4-, F-, Cl-, Br-, I-, and NO2-, thereby contributing significantly to solving the main problem of devices of this type proposed so far. Finally, the surfaces were characterized by atomic force microscopy, showing a strong anion-polymer layer interaction. Thus, this material can be proposed to be tested as a columbimetric nitrate sensor, highlighting the possibility of varying the area of the electrode and/or the thickness of the deposited polymeric layer and with it, the sensitivity of the device.
References
- M. J. Moorcroft, J. Davis, R. G. Compton, Talanta 54, 785, (2001)
- Y. Zhang, Y. Zhao, S. Yuan, H. Wang, C. He, Sens. Act. B-Chem. 185, 602, (2013)
- P. Miao, M. Shen, L. Ning, G. Chen, Y. Yin, Anal. Bioanaly. Chem. 399, 2407, (2011)
- A. Bebeselea, F. Manea, G. Burtica, L. Nagy, G. Nagy, Talanta 80, 1068, (2010)
- X.-H. Pham, C. A. Li, K. N. Han, B.-C. Huynh-Nguyen, T.-H. Le, E. Ko, J. H. Kim, G. H. Seong, Sens. Act. B-Chem. 193, 815, (2014)
- I. A. Wolff, A. E. Wasserman, Science 177, 15, (1972)
- M. E. E. Alahi, S. C. Mukhopadhyay, Sens. Act. A-Phys. 280, 210, (2018)
- E. Wierzbicka, J. Elem. 25, 97, (2020)
- M. E. E. Alahi, S. C. Mukhopadhyay, L. Burkitt, Sens. Act. B-Chem. 259, 753, (2018)
- Y. Fukao, Y. Kitazumi, K. Kano, O. Shirai, Anal. Sci. 34, 1373, (2018)
- A. Ivanova, K. Mikhelson, Sensors 18, 10, (2018)
- N. C. Lo, I. W. Sun, P. Y. Chen, J. Chin. Chem. Soc. 65, 982, (2018)
- M. Piek, R. Piech, B. Paczosa-Bator, J. Electrochem. Soc. 165, B60, (2018)
- H. Araar, M. Benounis, A. Direm, A. Touati, S. Atailia, H. Barhoumi, N. Jaffrezic-Renault, Mon. Chem. 150, 1737, (2019)
- A. Naser-Sadrabadi, H. R. Zare, Microchem J. 148, 206, (2019)
- Y. A. Shao, Y. T. Chen, P. Y. Chen, Electrochim. Acta 313, 488, (2019)
- A. M. Stortini, S. Fabris, G. Saorin, E. V. Falzacappa, L. M. Moretto, P. Ugo, Nanomat. 9, 12, (2019)
- N. Bommireddy, S. K. Palathedath, J. Electroanal. Chem. 856, 8, (2020)
- D. L. Li, T. Wang, Z. Li, X. B. Xu, C. Wang, Y. Q. Duan, Sensors 20, 35, (2020)
- Z. Mumtarin, M. M. Rahman, H. M. Marwani, M.A . Hasnat, Electrochim. Acta 346, 9, (2020)
- J. Y. Wang, P. Diao, Microchem J. 154, 6, (2020)
- U. Lange, N. V. Roznyatovskaya, V. M. Mirsky, Anal. Chim. Acta 614, 1, (2008)
- N. Gupta, S. Sharma, I. Ahmad Mir, D. Kumar, J. Sci. Ind. Res. 65, 549, (2006)
- M. A. Pardo, M. A. del Valle, F. R. Díaz, Polym. Bull. 72, 2189, (2015)
- M. A. Pardo, C. Navarrete, A. Ramírez, N. P. Barrera, S. Shultz, M. A. del Valle, F.R. Díaz, Int. J. Electrochem. Sci. 12, 3982, (2017)
- M. N. Bui, J. Brockgreitens, S. Ahmed, A. Abbas, Biosens. Bioelectron. 85, 280, (2016)
- G. A. Álvarez-Romero, M. E. Palomar-Pardavé, M. T. Ramírez-Silva, Anal. Bioanal. Chem. 387, 1533, (2007)
- M. Atmeh, B. E. Alcock-Earley, J. Appl. Electrochem. 41, 1341, (2011)
- T. Madasamy, M. Pandiaraj, M. Balamurugan, K. Bhargava, N. K. Sethy, C. Karunakaran, Biosens. Bioelectron. 52, 209, (2014)
- Y. Li, H. T. Han, D. W. Pan, P. Q. Zhang, J. Electrochem. Soc. 166, B1038 (2019)
- H. Essousi, H. Barhoumi, M. Bibani, N. Ktari, F. Wendler, A. Al-Hamry, O. Kanoun, J. Sens. 2019, 14, (2019)
- E. M. Bomar, G. S. Owens, G. M. Murray, Chemosens. 5, 8, (2017)
- P. Pu, M. Zhang, Y. H. Li, L. N. Zhang, H. Y. Ren, P. Kong, L. P. Pan, J. Electrochem. Sci. 11, 4779, (2016)
- M. R. Mahmoudian, Y. Alias, W. J. Basirun, M. W. Pei, F. Jamali-Sheini, M. Sookhakian, M. Silakhori, J. Electroanal. Chem. 751, 30, (2015)
- X. C. Tu, Y. Gao, R. Yue, Q. Lu, Y. K. Zhou, Z. X. Lu, Anal. Methods 4, 4182, (2012)

