PREDICTION OF PHYSICAL AND THERMODYNAMIC PROPERTIES OF ALIPHATIC ETHERS FROM MOLECULAR STRUCTURES BY MULTIPLE LINEAR REGRESSION
- Quantitative structure–property relationships (QSPR),
- Topological indices,
- Aliphatic ethers,
- Graph theory,
- Multiple linear regressions (MLR)
Copyright (c) 2017 F. Shafiei

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
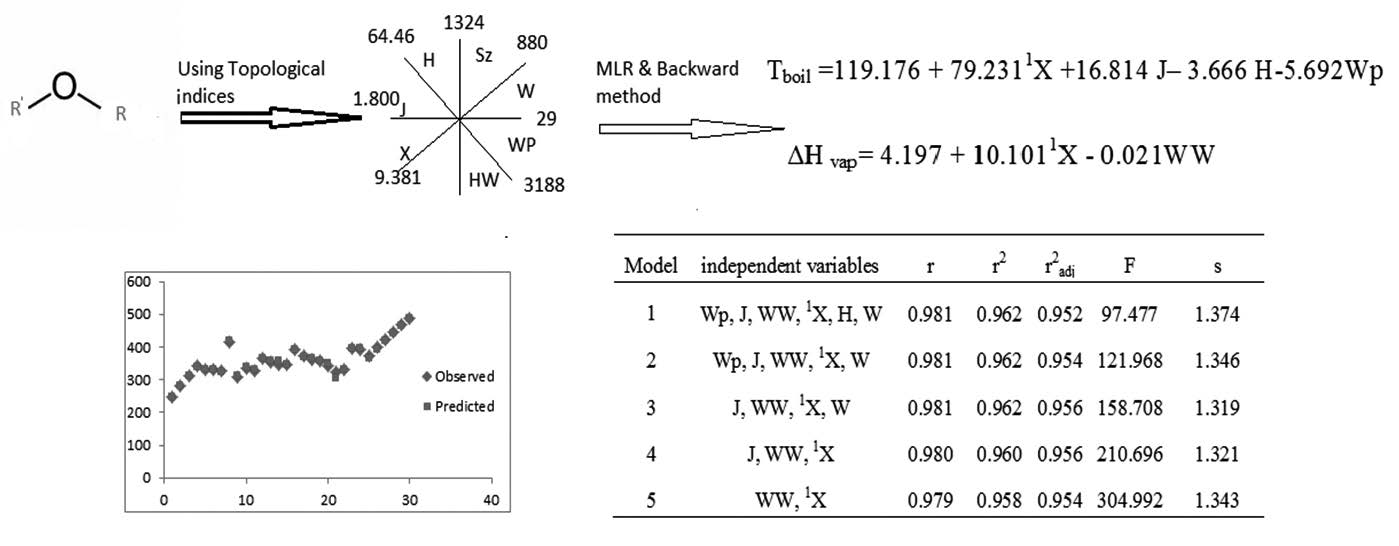
The interrelation of topological indices with enthalpy of vaporization at standard conditions ( DH° vap), and normal temperature of boiling point (Tboil /K) for series of aliphatic ethers has been investigated. For obtaining model for predicted target properties we have used multiple linear regression (MLR) techniques and followed Back ward regression analysis. The results have shown that combining the four descriptors (J, Wp, 1X, H) are included, with values of the correlation coefficient(r= 0.979), the standard error (s= 1.343 K),the Fisher –ratio(F= 304.992) could be used successfully for modeling and predicting the normal temperature of boiling points of 30 aliphatic ethers. The best model for estimating the enthalpy of vaporization of compounds are included two descriptors (WW, 1X), with values of the correlation coefficient(r=0.994), the standard error (s= 5.900kJ/mol ) and the Fisher –ratio(F= 558.789).
References
- M. Randic’, New J. Chem. 20,1001, (1996).
- I. Gutman, S. Klavžar, J. Chem. Inf. Comput. Sci. 35,1011, (1995).
- M. Randić, J. Am. Chem. Soc. 97,6609, (1975).
- D. J. Klein, I. Lukovits, I. Gutman, J. Chem. Inf. Comput. Sci. 35,50, (1995).
- F. Shafiei, Iranian. J. Math. Chem. 6,15, (2015).
- A. S. Bahjat, S. E. Rita, H. I. Sadigm, A. H. Kawkab, Am. J. Sci. 8,773, (2011).
- F. Ashrafi, R. Saadati, A. Behboodi Amlashi, African. J. Pure. Appl. Chem. 2,116,(2008).
- F. Shafiei , H. Hosseini , MATCH Commun. Math. Comput. Chem. 75,583, (2016).
- V. Sharma, R. Goswami, A.K .Madan, J. Chem. Inf. Comput. Sci. 37,273,(1997).
- G.L. Amidon, S.T. Anik, J. Pharm. Sci. 65,801,( 1976 ).
- V. G. Uryadov, А.I. Kourdioukov, N. Aristova, E. N. Vand Ofitserov, Butlerov Communications. 3,67,(2000).
- J. Hao, W. Xinghao, Y. Fen, W. Zunyao, J. Chemosphere. 80,665,(2010).
- K. Roy, D. KumarPal, C. Sengupta, Indian. J. Chem. 40,129,(2001).
- E. D. Nikitin, A. P. Popov, N.S. Bogatishcheva, J. Chem. Thermodynamics. 68,288, (2014).
- G. Espinosa, D. Yaffe, Y. Cohen, A .Arenas, F. Giralt, J. Chem .Inf. Comput. Sci. 40,859,(2000).
- A.R. Katritzky, V.S. Lobanov, M. Karelson, J. Chem. Inf. Comput. Sci. 38,28,(1998).
- Standard Reference Database Number 69; software available at http:// www.nist.gov
- M. Randić, J. Math. Chem. 7,155, (1991).
- A. T. Balaban, Chem. Phys. Lett. 89,399, (1982).
- K. C. Das, B. Zhou, N. Trinajstić, J. Math. Chem. 46,1369, (2009).
- Zhou, I. Gutman, Chem. Phys. Lett. 394,93, (2004).
- H. Deng, H. Xiao, F. Tang, MATCH Commun. Math. Comput. Chem. 63,257,(2010).
- M. Liu, B. Liu, MATCH Commun. Math. Comput. Chem. 66,293, (2011).
- Web search engine developed by Chem Axon; software available at http://www. Chemicalize. Org


