- Solution perchloric acid,
- Viscosity equations,
- Interaction parameters
Copyright (c) 2017 Renu Loshali, Narain Datt Kandpal

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
Viscosity ƞ and density ρ of aqueous concentrated perchloric acid 1.0moldm−3 to 9.0moldm−3 were measured at 297.65K. The viscosity data were used to calculate the value of A and B- coefficient of Jones- Dole equation. The value of B- coefficient is positive which suggests the strong ion- solvent interaction in aqueous perchloric acid. The three concentration regions of perchloric acid having value of B-coefficient (measure of ion-solvent interaction or structure making capacity) in the order 1.0 to 4.0moldm−3 < 4.0 to 6.0moldm−3 < 6.0 to 9.0moldm−3 has been proposed.
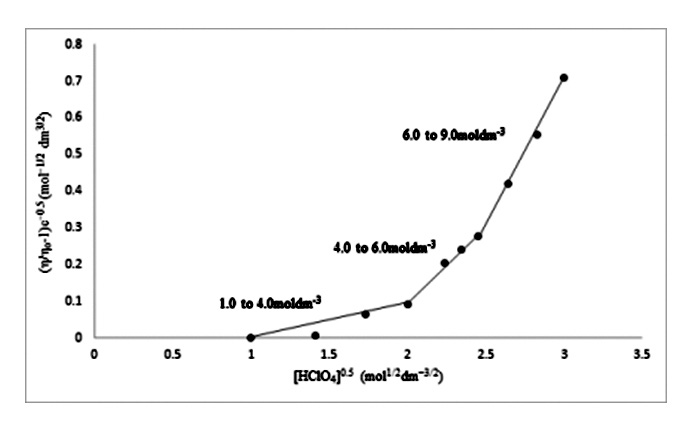
References
- A. I. Karelin, Z. I. Grigorovich, V. Ya Rosolovskii, Spectrochim Acta A: Molecular spectroscopy 31, 765 (1975).
- R. W. Duerst, J. Chem. Phys. 48, 2275 (1968).
- L. H. Jones, R. A. Penneman, J. Chem. Phys. 21, 542 (1953).
- P. S. Knapp, R. O. Waite, E. R. Malinowski, J. Chem. Phys. 49, 5459 (1968).
- M. Leuchs, G. Zundel, J. Chem. Soc. Faraday Trans. 2 74, 2256 (1978).
- J. W. Akitt, A. K. Covington, J. G. Freeman, T. H. Lilley, Trans. Faraday Soc. 65, 2701 (1969).
- G. V. Lagodzinskaya, G. B. Manelis, Z. K. Nikitine, V. I. Shestov, V. Ya Rosolovskii, Russ. Chem. Bull. 34, 708 (1985).
- A. I. Karelin, V. A. Tarasenko, Russ. Chem. Bull., Int. Ed. 52, 1959 (2003).
- A. S. Kertes, V. Kertes, J. Appl. Chem. 10, 287 (1960).
- H. J. Bakker, M. F. Kropmann, A. W. Omta, J. Phys.: Condens. Matter 17, S3215 (2005).
- H. S. Harned, N. N. T. Samaras, J. Am. Chem. Soc. 54, 9 (1932).
- N. H. El Hammamy, A. A. Hasanein, M. F. Amira, F. M. ABD El Halim, J. Indian Chem. Soc. LXI, 512 (1984).
- A. D. Aprano, J. Phys. Chem. 75, 3290 (1971).
- P. G. Traverso, Can. J. Chem. 62, 153 (1984).
- E. R. Nightingale, B. E. Conway, R. G. Barradas, Chemical Physics of Ionic Solution, Wiley, New York, 1966.
- L. Grunberg, A. H. Nissan, Nature 164, 799 (1949).
- R. K. Hind, E. McLaughlin, A. R. Ubbelohde, Trans. Faraday Soc. 56, 328 (1960).
- R. Loshali, B. Chandra, N. Sah, N. D. Kandpal, Int. J. Chem. Sci. 12, 1439 (2014).
- H. Erying, M. S. John, Significant liquid structure, Wiley, New York, 1969.


