CHEMICAL CHARACTERIZATION OF LIGNANS FROM ARAUCARIA ARAUCANA A NATIVE CONIFER OF CHILE AND EVALUATION OF THEIR CYTOTOXICITY AND ANTIOXIDANT ACTIVITIES

- Araucaria araucana,
- lignan,
- eudesmin,
- DPPH activity,
- cytotoxic activity
Copyright (c) 2020 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
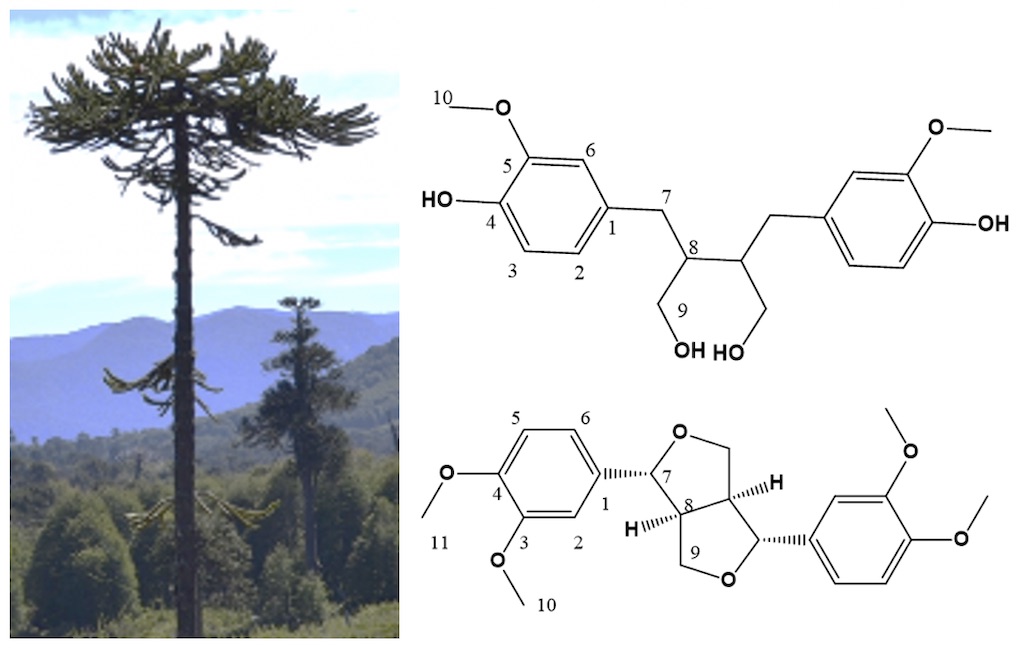
Araucaria araucana is a native conifer of Chile commonly called Araucaria. The knots of Araucaria are extremely hard wood and highly rot-resistant, they can be found in the forest decades after that the tree has dead and decomposed. Here we report the phytochemical characterization of different parts of the Araucaria as stemwood, branch and knots, founding a remarkable difference in the content of extractables in these parts, as well as the lignan composition, which is higher in knots than in branches or stemwood. Eudesmin was isolated and crystallized from organic extract of knots, its structure was determinate by NMR; moreover, secoisolariciresinol, lariciresinol and matairesinol were identified by GCMS and HPLC in contrast to standards, and quantified in stemwood, branch and knots. The results showed that secoisolariciresinol is the main lignan with 45.77 mg g-1, followed by eudesmin with 22.68 mg g-1, lariciresinol 4.57 mg g-1 and matairesinol with 1.19 mg g-1. The antioxidant activity in terms of DPPH assay showed that knotwood extract displays the higher activity, meanwhile that eudesmin did not displays activity in DPPH assay. The cytotoxic activity against SHSY5Y neuroblastoma and P3X myeloma cell lines, revelated a moderate activity of extracts, while eudesmin did not showed activity.
References
- Vermerris, W., Nicholson, R.: Phenolic Compound Biochemistry. Springer, Dordrecht ; London (2008)
- Kebbi-Benkeder, Z., Colin, F., Dumarçay, S., Gérardin, P.: Quantification and characterization of knotwood extractives of 12 European softwood and hardwood species. Ann. For. Sci. 72, 277–284 (2015). https://doi.org/10.1007/s13595-014-0428-7
- Valette, N., Perrot, T., Sormani, R., Gelhaye, E., Morel-Rouhier, M.: Antifungal activities of wood extractives. Fungal Biol. Rev. 31, 113–123 (2017). https://doi.org/10.1016/j.fbr.2017.01.002
- Trapp, S., Croteau, R.: Defensive resin biosynthesis in conifers. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 689–724 (2001). https://doi.org/10.1146/annurev.arplant.52.1.689
- Kebbi-Benkeder, Z., Dumarçay, S., Touahri, N., Manso, R., Gérardin, P., Colin, F.: Les noeuds: un bois méconnu et une source importante de composés extractibles. Biol. Écologie. (2016)
- Willfor, S., Nisula, L., Hemming, J., Reunanen, M., Holmbom, B.: Bioactive phenolic substances in industrially important tree species. Part 1: Knots and stemwood of different spruce species. Holzforschung. 58, (2004). https://doi.org/10.1515/HF.2004.052
- Belt, T., Hänninen, T., Rautkari, L.: Antioxidant activity of Scots pine heartwood and knot extractives and implications for resistance to brown rot. Holzforschung. 71, (2017). https://doi.org/10.1515/hf-2016-0232
- Anderegg, R.J., Rowe, J.W.: Lignans, the Major Component of Resin from Araucaria angustifolia Knots. Holzforschung. 28, 171–175 (1974). https://doi.org/10.1515/hfsg.1974.28.5.171
- Ohashi, H., Kawai, S., Sakurai, Y., Yasue, M.: Norlignan from the knot resin of Araucaria angustifolia. Phytochemistry. 31, 1371–1373 (1992). https://doi.org/10.1016/0031-9422(92)80293-N
- Willför, S.M., Smeds, A.I., Holmbom, B.R.: Chromatographic analysis of lignans. J. Chromatogr. A. 1112, 64–77 (2006). https://doi.org/10.1016/j.chroma.2005.11.054
- Hatano, T., Edamtsu, R., Hiramatsu, M., Mori, A., Fujita, Y., Yasuhara, T., Yoshida, T., Okuda, T.: Effects of the Interaction of Tannins with Co-existing Substances. VI. : Effects of Tannins and Related Polyphenols on Superoxide Anion Radical, and on 1,1-Diphenyl-2-picrylhydrazyl Radical. Chem Pharm Bull. 37, 2016–2021 (1989)
- Dewanjee, S., Gangopadhyay, M., Bhattacharya, N., Khanra, R., Dua, T.K.: Bioautography and its scope in the field of natural product chemistry. J. Pharm. Anal. 5, 75–84 (2015). https://doi.org/10.1016/j.jpha.2014.06.002
- Garzoli, S., Masci, V.L., Ovidi, E., Turchetti, G., Zago, D., Tiezzi, A.: Chemical Investigation of a Biologically Active Schinus molle L. Leaf Extract. J. Anal. Methods Chem. 2019, 1–6 (2019). https://doi.org/10.1155/2019/8391263
- Yamamoto, S., Otto, A., Simoneit, B.R.T.: Lignans in resin of Araucaria angustifolia by gas chromatography/mass spectrometry. J. Mass Spectrom. 39, 1337–1347 (2004). https://doi.org/10.1002/jms.726
- Yamamoto, S., Cox, R.E., Simoneit, B.R.: Gas Chromatography/Mass Spectrometry of the Lignans in Resin of Callitris preissii. J. Mass Spectrom. Soc. Jpn. 58, 195–209 (2010)
- Parhoodeh, P., Rahmani, M., Hashim, N.M., Sukari, M.A., Cheng Lian, G.E.: Lignans and Other Constituents from Aerial Parts of Haplophyllum Villosum. Molecules. 16, 2268–2273 (2011). https://doi.org/10.3390/molecules16032268
- DellaGreca, M., Zuppolini, S., Zarrelli, A.: Isolation of lignans as seed germination and plant growth inhibitors from Mediterranean plants and chemical synthesis of some analogues. Phytochem. Rev. 12, 717–731 (2013). https://doi.org/10.1007/s11101-013-9311-7
- Céspedes A, C.L., Avila, J.G., Marin, J.C., Domínguez L, M., Torres, P., Aranda, E.: Chapter 1 Natural compounds as antioxidant and molting inhibitors can play a role as a model for search of new botanical pesticides. In: Carpinella, M.R. and M.C. (ed.) Advances in Phytomedicine. pp. 1–27. Elsevier (2006)
- Landete, J.M.: Plant and mammalian lignans: A review of source, intake, metabolism, intestinal bacteria and health. Food Res. Int. 46, 410–424 (2012). https://doi.org/10.1016/j.foodres.2011.12.023
- Yatkin, E., Polari, L., Laajala, T.D., Smeds, A., Eckerman, C., Holmbom, B., Saarinen, N.M., Aittokallio, T., Mäkelä, S.I.: Novel Lignan and Stilbenoid Mixture Shows Anticarcinogenic Efficacy in Preclinical PC-3M-luc2 Prostate Cancer Model. PLoS ONE. 9, e93764 (2014). https://doi.org/10.1371/journal.pone.0093764
- Balík, J., Híc, P., Kulichová, J., Novotná, P., Tříska, J., Vrchotová, N., Strohalm, J., Lefnerová, D., Houška, M.: Musts with Increased Lignan Content Through Addition of Lignan Extracts. Food Bioprocess Technol. 10, 1367–1373 (2017). https://doi.org/10.1007/s11947-017-1911-6
- Josef, B., Pavel, H., Jana, K., Pavla, N., Jan, T., Naděžda, V., Jan, S., Milan, H.: Wines with Increased Lignan Content by the Addition of Lignan Extracts. Czech J. Food Sci. 34, 439–444 (2016). https://doi.org/10.17221/575/2015-CJFS
- Calvo-Flores, F.G., Dobado Jiménez, J.A., Garcia, J.I., Martín-Martínez, F.J.: Lignin and lignans as renewable raw materials: chemistry, technology and applications. John Wiley and Sons, Inc, Chichester, West Sussex, United Kingdom (2015)
- Donoso, C.A., Becerra, J., Bittner, M., Elissetche, J.P., Freer, J., Mendoza, R., Sterner, O., Silva, M.: Allelochemicals and natural durability in Chilean Cupressaceae heartwoods. Allelopathy J. 21, 119–132 (2008)
- Kebbi-Benkeder, Z., Manso, R., Gérardin, P., Dumarçay, S., Chopard, B., Colin, F.: Knot extractives: a model for analysing the eco-physiological factors that control the within and between-tree variability. Trees. (2017). https://doi.org/10.1007/s00468-017-1573-z
- Lim, S., Grassi, J., Akhmedjanova, V., Debiton, E., Balansard, G., Beliveau, R., Barthomeuf, C.: Reversal of P-Glycoprotein-Mediated Drug Efflux by Eudesmin from Haplophyllum perforatum and Cytotoxicity Pattern versus Diphyllin, Podophyllotoxin and Etoposide. Planta Med. 73, 1563–1567 (2007). https://doi.org/10.1055/s-2007-993754
- Jiang, L.-L., Sun, B.-R., Zheng, C., Yang, G.-L.: The antitumour effects of eudesmin on lung cancer by inducing apoptosis via mitochondria-mediated pathway in the tumour cells. Pharm. Biol. 55, 2259–2263 (2017). https://doi.org/10.1080/13880209.2017.1401647
- Yu, M., Li, Y., Li, M., Lu, D.: Eudesmin exerts antitumor effects by down-regulating EZH2 expression in nasopharyngeal carcinoma cells. Chem. Biol. Interact. 307, 51–57 (2019). https://doi.org/10.1016/j.cbi.2019.04.028

