TWO NOVEL TCPP PORPHYRINIC COMPOUNDS: IN SITU SYNTHESES, CHARACTERIZATION AND REACTION MECHANISM
- Esterification,
- In situ,
- Porphyrin,
- TCPP,
- Zinc
Copyright (c) 2017 Ding-Wa Zhang, Wen-Tong Chen

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
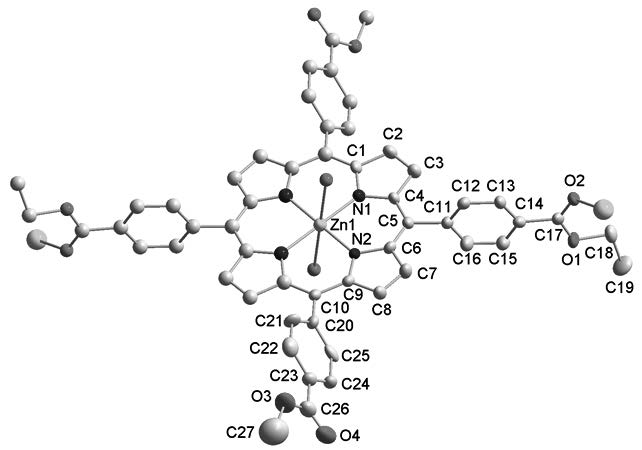
Two novel porphyrinic compounds, {Zn[TCPP(CH2CH3)2(CH3)2]}n (1) and TCPP(CH3)4 (2) (TCPP = meso-tetra(4-carboxyphenyl)porphyrin), with the TCPP(CH2CH3)2(CH3)2 and TCPP(CH3)4 generated in situ, have been synthesized via a solvothermal reaction and structurally characterized by single-crystal X-ray diffraction. Compound 1 is characteristic of a two-dimensional (2-D) coordination polymer, based on the zinc ion coordinating to four nitrogen atoms and two oxygen atoms. Compound 1 possesses a large void space (220 Å3) corresponding to 4.6% of the unit-cell volume. Compound 2 is characterized by an isolated structure. The reaction mechanism of preparing both compounds was explored. The photoluminescence properties, FT-IR, UV-vis absorption spectra, fluorescence lifetime and fluorescence quantum yield of TCPP were also reported.
References
- Lu J. Y.. Coord. Chem. Rev., 246, 327–347 (2003).
- Chen X. M., Tong M. L.. Acc. Chem. Res., 40, 162–170 (2007).
- Benny P. D., Fugate G. A., Barden A. O., Morley J. E., Silva-Lopez E., Twamley B.. Inorg. Chem., 47, 2240–2242 (2008).
- Chen W.-T.. J. Chem. Res., 405–407 (2011).
- Chen W.-T., Hu L.. Acta Chim. Slov., 58, 167–170 (2011).
- Chen W.-T., Liu D.-S., Luo Z.-G., Chen H.-L., Liu J.-H.. J. Chem. Res., 491–493 (2011).
- Silva A. M. G., Tomé A. C., Neves M. G. P. M. S., Cavaleiro J. A. S.. Tetrahedron Lett., 41, 3065–3068 (2000).
- Tomé A. C., Lacerda P. S. S., Neves M. G. P. M. S., Cavaleiro J. A. S.. Chem. Commun., 1199–1200 (1997).
- Cavaleiro J. A. S., Neves M. G. P. M. S., Tomé A.C.. Arkivoc, 14, 107– 130 (2003).
- Chen W.-T., Liu D.-S., Xu Y.-P., Luo Q.-Y., Pei Y.-P.. Luminescence, 31, 158–163 (2016).
- Zhang X., Chen W.-T., Suenobu T., Fukuzumi S., Wang M.-S., Guo G.-C.. J. Porphyr. Phthalocya., 19, 1225–1231 (2015).
- Pei Y.-P., Huang J.-G., Chen H.-L., Kuang H.-M., Zhou J., Yang Y.-X., Chen W.-T.. J. Porphyr. Phthalocya., 19, 1140–1146 (2015).
- Chen W.-T., Huang J.-G., Lei X.-Y., Hu R.-H., Pei Y.-P., Yang Y.-X., Zhou J.. J. Iran. Chem. Soc., 13, 95–101 (2016).
- Rigaku, CrystalClear Version 1.35, Rigaku Corporation, Tokyo, Japan (2002).
- Siemens, SHELXTLTM Version 5 Reference Manual, Siemens Energy & Automation Inc., Madison, Wisconsin, USA (1994).
- Shultz D. A., Mussari C. P., Ramanathan K. K., Kampf J. W.. Inorg. Chem., 45, 5752–5759 (2006).
- Gros C. P., Brisach F., Meristoudi A., Espinosa E., Guilard R., Harvey P. D.. Inorg. Chem., 46, 125–135 (2007).
- Yang F.-A., Guo C.-W., Chen Y.-J., Chen J.-H., Wang S.-S., Tung J.-Y., Hwang L.-P., Elango S.. Inorg. Chem., 46, 578–585 (2007).
- Muniappan S., Lipstman S., Goldberg I.. Acta Crystallogr., Sect. C., 62, m495–m497 (2006).
- Muniappan S., Lipstman S., Goldberg I.. Acta Crystallogr., Sect. C., 62, m140–m143 (2006).


