EFFECTS OF PHYSICAL AND CHEMICAL MODIFICATION ON BIOLOGICAL ACTIVITIES OF CHITOSAN/ CARBOXYMETHYLCELLUSE BASED HYDROGELS
- Chitosan,
- Carboxymethylcellulose,
- Hydrogels,
- Swelling,
- Biological activities
Copyright (c) 2017 Saida Benghanem, Asma Chetouani, Meriem Elkolli, Mahmoud Bounekhel, Djafar Benachour

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
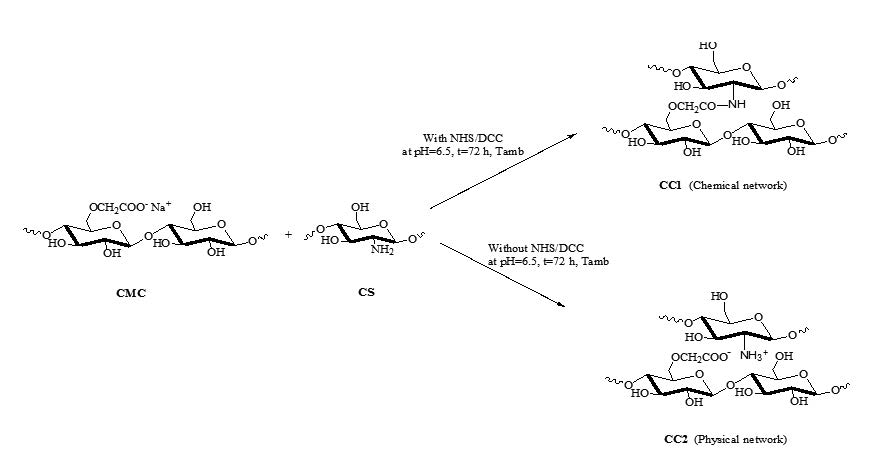
Hydrogels network based on carboxymethylcellulose (CMC) and chitosan (CS), CC1 and CC2, have been prepared respectively in the presence or without the crosslinking agents [(N-hydroxysuccinimide (NHS)/N,N’-dicyclohexylcarbodiimide (DCC)] and characterized by FT-IR.
The swelling behavior in distilled water at 25°C and biological activities have been investigated. CC1 hydrogel revealed higher potential swelling than CC2. Hydrogels showed to possess an important antioxidant activity equal to 66.67% for CC1 and 57.27% for CC2, to scavenge hydroxyl radicals at 2mg/mL. And the values of their reducing power were approximately 53% and 57%.
From the hemolytic potential test the obtained materials were hemo-compatible. The anti-inflammatory activity exhibited that hydrogels were able to protect albumin from denaturation.
References
- G. Buhus, M. Popa, J. Desbrieres, J. Bioact. Compat. Polym. 24, 525, (2009).
- S.R. Van Tomme, G. Storm, W.E. Hennink, Int. J. Pharm. 355, 1, (2008).
- N. Saha, A. Saarai, N. Roy, T. Kitano, P. Saha, J. Biomater. Nanobiotechnol. 2, 85, (2011).
- L.G. Gómez-Mascaraque, J.A. Méndez, M. Fernández-Gutiérrez, Acta. Biomater. 10, 798, (2014).
- A. Teotia, S. Ikram, B. Gupta, Polym. Bull. 69, 175, (2012).
- Y. Li, S. Zhang, X. Meng, X. Chen, G. Ren, Carbohydr. Polym. 83,130, (2011).
- V. Balan, L. Verestiuc, Eur. Polym. J. 53, 171, (2014).
- L. Weng, P. Rostamzadeh, N. Nooryshokry, H.C. Le, J. Golzarian, Acta. Biomater. 9, 6823,(2013).
- M. Dash, F. Chiellini, R.M. Ottenbrite, E. Chiellini, Prog. Polym. Sci. 36, 981, (2011).
- A. Di Martino, M. Sittinger, M.V. Risbud, Biomaterials 26, 5983, (2005).
- D.R. Biswal, R.P. Singh, Carbohydr. Polym. 57, 379, (2004).
- L. Fan, M. Peng, X. Zhou, H. Wu, J. Hu, W. Xie, S. Liu, Carbohydr. Polym. 112, 32, (2014).
- L. Fan, X. Zhou, P. Wu, W. Xie, H. Zheng, W. Tan, Q. Li, Int. J. Biol. Macromol. 66, 245, (2014).
- X.H. Zhao, X.W He, S.Q Xie, L.S. Yang, Appl. Mech. Mater. 20, 1157, (2010).
- H. Chen, M. Fan, J. Bioact. Compat. Polym. 22, 475, (2007).
- A.I.R. Matute, A. Cardelle-Cobas, A.B García-Bermejo, A. Montilla, A. Olano, N. Corzo, Food Hydrocoll. 33, 245, (2013).
- B. Hoffmann, D. Seitz, A. Mencke, A. Kokott, G. Ziegler, J. Mater. Sci. Mater. Med. 20, 1495, (2009).
- N. Dhar, S.P. Akhlaghi, K.C. Tam, Carbohydr. Polym. 87, 101, (2012).
- C. Delattre, G. Pierre, C. Gardarin, M. Traikia, R. Elboutachfaiti, A. Isogai, P. Michaud, Carbohydr. Polym. 116, 34, (2015).
- G.C. Yen, H.Y. Chen, J. Agric. Food. Chem. 43, 27, (1995).
- R. Subramanian, P. Subbramaniyan, V. Raj, SpringerPlus. 2, 1, (2013).
- L. Fan, Y. Sun, W. Xie, H. Zheng, S. Liu, J. Biomater. Sci. Polym. Ed. 23, 2119, (2012).
- F. Alhakmani, S. Kumar, S.A. Khan, Asian. Pac. J. Trop. Biomed. 3, 623, (2013).
- H. Kono, Carbohydr. Polym. 106, 84, (2014).
- F.A. Al-Sagheer, E.I. Ibrahim, K.D. Khalil, Eur. Polym. J. 58, 164, (2014).
- S.M. Prabhu, S. Meenakshi, J. Water Process. Eng. 2, 96, (2014).
- M. Ibrahim, A.A. Mahmoud, O. Osman, A. Refaat, E.S.M. El-Sayed, Spectrochim. Acta. A Mol. Biomol. Spectrosc. 77, 802, (2010).
- A. Chetouani, M. Elkolli, M. Bounakhel, B. Djaafer, J. Chil. Chem. Soc. 59, 2279, (2014).
- H. Bidgoli, A. Zamani, M.J. Taherzadeh, Carbohydr. Res. 345, 2683, (2010).
- E. Rollet-Labelle, M.J. Grange, C. Elbim, C. Marquetty, M.A. Gougerot- Pocidalo, C. Pasquier, Free Radic. Biol. Med. 24, 563, (1998).
- J.Y. Je, S.K. Kim, Biorgan. Med. Chem. 14, 5989, (2006).
- H. Osman, R. Nasarudin, S.L Lee, Food Chem. 86, 41, (2004).
- I.F. Benzie, J.J. Strain, Anal. Biochem. 239, 70, (1996).
- Y.C. Chung, C.T. Chang, W.W Chao, C.F. Lin, S.T. Chou, J. Agric. Food. Chem. 50, 2454, (2002).
- W. Dameshek, R. Schwartz, Ann. N. Y. Acad. Sci. 77, 589, (1959).
- S. Chandra, P. Chatterjee, P. Dey, S. Bhattacharya, Asian Pac. J. Trop. Biomed. 2, S178, (2012).
- Y.M. Bagad, A.R. Umarkar, A.U. Tatiya, S. J. Surana, J. Pharm. Res. 4, 1132, (2011).


