DEVELOPMENT, COMPUTATIONAL STUDIES AND VALIDATION OF SPECTROPHOTOMETRIC METHOD OF METFORMIN HYDROCHLORIDE IN PHARMACEUTICAL FORMULATIONS

- Spectrophotometer,
- Metformin,
- Method development,
- Validation,
- Tablets
- Computational ...More
Copyright (c) 2020 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
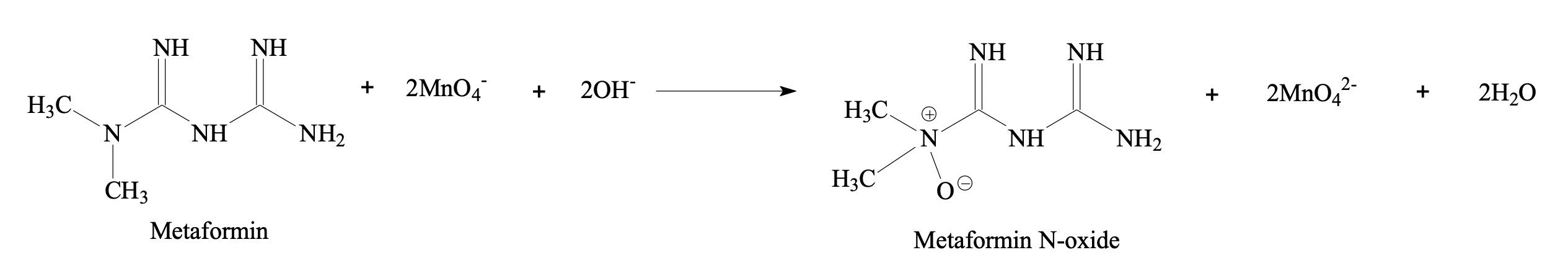
Simple, fast and sensitive spectrophotometric method was developed for the determination of metformin in pharmaceutical formulations. The spectrophotometric procedure was based on the oxidation of metformin with potassium permanganate in alkaline medium. The developed method was also validated according to International Conference on Harmonization guidelines parameters like linearity, accuracy, precision, limit of detection and quantitation. Studies were conducted to investigate different parameters involved in color developments and optimized. The pure drug of metformin was extracted from pharmaceutical dosage form. Extraction procedure was developed, and possible reaction mechanism proposed in the manuscript. The IR spectrum of the extracted metformin was taken and compared with the simulated IR spectrum obtained from the density functional theory calculation. The vibrational assignment of the modes was done based on potential energy distribution.
References
- REFERENCES
- ADA. “Standards of medical care for patients with diabetes mellitus (Position Statement).” Diabetes Care 26, 2003 (Suppl. 1): S33–S50.
- Ahir, KB, EM Patelia and A Shah. “Simultaneous estimation of Metformin hydrochloride and Repaglinide in pharmaceutical formulation by HPTLC densitometry method.” Journal of Chromatography Separation Techniques. 2013;4:1000166.
- Ahmed, G, AS Hani and CR Dass. “Development and validation of a new analytical HPLC method for simultaneous determination of the antidiabetic drugs, metformin and gliclazide.” Journal of Food and Drug Analysis. 2019;27(1):315–322.
- Ambadas, RR and BS Ravindranath. “Estimation of Metformin hydrochloride by UV spectrophotometric method in pharmaceutical formulation.” World Journal of Pharmaceutical Sciences. 2014;2(12):1841-45.
- Amruta, BL, RG Minal and SD Sawant. “Simultaneous UV spectrophotometric method for estimation of Sitagliptin phosphate and Metformin hydrochloride in bulk and tablet dosage form.” Der Pharma Chemica. 2012;4(3):854-9.
- Audumbar, DM, M Seeta, A Tamboli and R Bathe. “Simultaneous UV spectrophotometric methods for estimation of Metformin HCl and Glimepiride in bulk and tablet dosage form.” International Journal of Advances in Pharmaceutics. 2015;4(6):117–24.
- Bailey CJ and RC Turner. “Metformin.” The New England Journal of Medicine. 1996; 334(9):574–579.
- Basavaiah K and N Rajendraprasad. “Selective spectrophotometric determination of Metformin hydrochloride in pharmaceuticals and urine using two nitrophenols as chromogenic agents.” Analytical Bioanalytical Chemistry Research. 2017;4(1):41-51.
- Becke AD. “Density-functional thermochemistry. III. The role of exact exchange. The Journal of Chemical Physics. 1993;98(7):5648-53.
- Bhaskar, R, R Bhaskar, MK Sagar, V Saini and KM Bhat. “UV-spectrophotometric assisted chemometric methods for the simultaneous determination of Metformin hydrochloride and Gliclazide in pharmaceutical formulations.” Pharmaceutica Analytica Acta. 2012;3(4):1000158.
- CDER. “Department of Health and Human Services: Center for Veterinary Medicine (CVM). Food and Drug Administration of the United States, Guidance for industry–Bioanalytical Method Validation.” 2001.
- Chhetri, HP, T Panna and AV Schepdael. “Simple HPLC-UV method for the quantification of Metformin in human plasma with one step protein precipitation.” Saudi Pharmaceutical Journal. 2014;22(5):483–487.
- Chalikwar, SS, DR Shah and PS Jain. “Estimation of Metformin hydrochloride in bulk and granules by ion-pair liquid chromatography.” Analytical Chemistry: An Indian Journal. 2016;16(15):115.
- Dange, YD, SM Honmane, SD Bhinge, VR Salunkhe and DR Jadge. “Development and validation of uv-spectrophotometric method for estimation of Metformin in bulk and tablet dosage form.” Indian Journal of Pharmaceutical Education and Research. 2017;51(4S):S754-S60.
- Darshana, KM, PB Parejiya and BH Patel. “A Simple and sensitive HPTLC method for simultaneous determination of Metformin hydrochloride and Sitagliptin phosphate in tablet dosage form.” Journal of Chemistry. 2013;Volume 2013:139561.
- DeFronzo, RA, N Barzilai and DC Simonson. “Mechanism of Metformin action in obese and lean noninsulin-dependent diabetic subjects.” The Journal of Clinical Endocrinology & Metabolism. 1991;73(6):1294–1301.
- Farha KO and RA Nief. “Spectrophotometric determination of Metformin hydrochloride via oxidative coupling reaction with 1-naphthol in pharmaceutical and environmental water sample.” Iraqi National Journal of Chemistry. 2012;46:161-170.
- Frisch, MJ, GW Trucks, HB Schlegel, GE Scuseria, MA Robb, JR Cheeseman, G Scalmani, V Barone, B Mennucci, GA Petersson, H Nakatsuji, M Caricato, X Li, HP Hratchian, AF Izmaylov, J Bloino, G Zheng, JL Sonnenberg, M Hada, M Ehara, K Toyota, R Fukuda, J Hasegawa, M Ishida, T Nakajima, Y Honda, O Kitao, H Nakai, T Vreven, JA Montgomery, JE Peralta, F Ogliaro, M Bearpark, JJ Heyd, E Brothers, KN Kudin, VN Staroverov, R Kobayashi, J Normand, K Raghavachari, A Rendell, JC Burant, SS Iyengar, J Tomasi, M Cossi, N Rega, JM Millam, M Klene, JE Knox, JB Cross, V Bakken, C Adamo, J Jaramillo, R Gomperts, RE Stratmann, O Yazyev, AJ Austin, R Cammi, C Pomelli, JW Ochterski, RL Martin, K Morokuma, VG Zakrzewski, GA Voth, P Salvador, JJ Dannenberg, S Dapprich, AD Daniels, O Farkas, JB Foresman, JV Ortiz, J Cioslowski, and DJ Fox. “Gaussian 09, Revision 02, Gaussian, Inc., Wallingford CT, 2009.
- Ganesh, K, G Nikitha, D Sireesha and B Vasudha. “Development and validation of UV spectrophotometric method for simultaneous estimation of Metformin and Glipizide in tablet dosage form.” International Journal of Applied Pharmaceutical Sciences and Research. 2016;1(2):56-9.
- Gazala, MBH, AAA Ashraf, S Bahruddin, M Ahmad and MS Salizawati. “Method validation for determination of Metformin hydrochloride in pharmaceutical formulations by capillary electrophoresis with capacitively coupled contactless conductivity detection.” Chemical Science International Journal. 2019; 26(1):CSIJ.47236.
- ICH. “International Conference on the Harmonization of the Technical Requirements for Registration of Pharmaceuticals for Human Use, Q2A: Validation of Analytical Methods.” 1994.
- ICH. “Analytical Validation – Methodology, Q2A.” 1996.
- Jamroz MH. “Vibrational Energy Distribution Analysis.” VEDA 4 Program Warsaw, 2004.
- Karim, R, N Poly and R Banoo. “Development and validation of UV Spectroscopic method for the determination of Metformin hydrochloride in tablet dosage form.” International Journal of Pharmaceutical Sciences and Research. 2012;3(9):3170-74.
- Krentz AJ and CJ Bailey. “Oral antidiabetic agents: current role in type 2 diabetes mellitus.” Drugs. 2005;65(3):385–411.
- Lakshmi, KS, T Rajesh and S Shrinivas. “Simultaneous determination of Metformin and Pioglitazone by reversed phase HPLC in pharmaceutical dosage forms.” International Journal of Pharmacy and Pharmaceutical Sciences. 2009;1(2):162–166.
- Lee, C, W Yang and RG Parr. “Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Physical Review B. 1988;37(2):785-89.
- Mahmoud, RS, K Naghmeh and M Khakpour. “Simultaneous spectrophotometric determination of Metformin hydrochloride and Glibenclamide in binary mixtures using combined discrete and continuous wavelet transforms.” Analytical Sciences. 2011;27(10):1037–41.
- Mary, RY, V Sudha and HAK Kumar. “A validated high performance liquid chromatography method for the determination of Metformin in human plasma and its application to pharmacokinetic study.” Chromatography and Separation Techniques Journal. 2019;2(1):119.
- Minal, H, L Sameer, G Valmik, C Vitthal and A Khomne. “Ultraviolet-spectrophotometric method for simultaneous estimation of Dapagliflozin propanediol and Metformin hydrochloride.” International Research Journal of Pharmacy. 2019;10(4):90–4.
- Mubeen G and N Khalikha. “Spectrophotometric method for analysis of Metformin hydrochloride.” Indian Journal Pharmaceutical Sciences. 2009;71(1):100-2.
- Musi, N, MF Hirshman, J Nygren, M Svanfeldt, P Bavenholm, O Rooyackers. G Zhou, JM Williamson, O Ljunqvist, S Efendic, DE Moller, A Thorell and LJ Goodyear. “Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes.” Diabetes. 2002;51(7):2074–81.
- Musutova, M, M Elkalaf, N Klubickova, M Koc, S Povysil, J Rambousek, B Volckaert, F Duska, MD Trinh, M Kalous, J Trnka, K Balusikova, J Kovar and J Polak. “The effect of Hypoxia and Metformin on fatty acid uptake, storage, and oxidation in L6 differentiated Myotubes.” Front Endocrinol (Lausanne). 2018;9:616.
- Nafisur, R, R Habibur, SM Haque. “Kinetic spectrophotometric method for the determination of perindopril erbumine in pure and commercial dosage forms.” Arabian Journal of Chemistry. 2017; 10(Supplement 1):S831-8.
- Regina, A, F Pitasari and Rusdi. “Development and validation of TLC densitometry method for simultaneous determination of Metformin HCl and Glibenclamide in tablets dosage form.” Journal of Chemical and Pharmaceutical Research. 2015;7(9S), 159-164, 2015.
- Rena, G, E Pearson and K Sakamoto. “Molecular mechanism of action of Metformin: old or new insights?” Diabetologia 2013;56(9): 1898-906.
- Sahar, A, Y Umar and I Mokhtar. "Conformational and vibrational analysis of 2- , 3- and 4-Pyridinecarbonyl chloride using DFT." Zeitschrift für Physikalische Chemie. 2016;230(5-7):867-82.
- Scott AP and L Radom. “Harmonic vibrational frequencies: An evaluation of Hartree−Fock, Møller−Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors.” The Journal of Physical Chemistry. 1996;100(41):16502-13.
- Serap SA and Y Berna. “Derivative spectrophotometric and isocratic high-performance liquid chromatographic methods for simultaneous determination of Repaglinide and Metformin hydrochloride in pharmaceutical preparations.” American Journal of Analytical Chemistry. 2017; 8(9):541-552.
- Silverstein RM and FX Webster. “Spectrometric identification of organic compounds.” Sixth edition, John Wiley and Sons, Inc. USA, 1998.
- Srinivasa, RP, RP Nageswara, R Gajula and G Venkateswarlu. “Simultaneous determination of Atorvastatin, Metformin and Glimepiride in human plasma by LC–MS/MS and its application to a human pharmacokinetic study.” Journal of Pharmaceutical Analysis. 2013;3(1):9–19.
- Telny, TC, YR Padmanabha and N Devanna. “Simultaneous spectrophotometric estimation of Metformin hydrochloride and Glipizide in tablet dosage forms.” International Journal of PharmTech Research. 2011;3(4):2064–67.
- Umapathi, P, J Ayyappan and SD Quine. “Quantitative determination of Metformin hydrochloride in tablet formulation containing croscarmellose sodium as disintegrant by HPLC and UV spectrophotometry.” Tropical Journal of Pharmaceutical Research. 2012;11(1): 107-116.
- Umar Y. “Density functional theory calculations of the internal rotation and vibrational spectra of 2-, 3-, 4- formyl pyridine.” Spectrochemica Acta A: Molecular and Biomolecular Spectroscopy. 2009;71(5):1907-13.
- Umar Y and S Abdalla. “DFT study of the molecular structure, conformational preference, HOMO, LUMO, and vibrational analysis of 2-, and 3-Furoyl Chloride.” Journal of Solution Chemistry. 2017;46(4):741-58.
- Umar, Y, J Tijani and S Abdalla. “Conformational stabilities, rotational barriers, and vibrational spectra of 2-Pyrrolecarboxaldehyde and 3-Pyrrolecarboxaldehyde calculated using density functional theory.” Journal of Structural Chemistry. 2019;60(2):186–97.
- Umar, Y, J Tijani and S Abdalla. "Density functional theory studies of conformational stabilities and rotational barriers of 2- and 3-Thiophenecarboxaldehydes." Journal of Structural Chemistry. 2016;57(8):1543-53.
- Umar, Y, N. Abu-Thabit, P Jerabek and P Ramasami. “Experimental FTIR and theoretical investigation of the molecular structure and vibrational spectra of acetanilide using DFT and dispersion correction to DFT.” Journal of Theoretical and Computational Chemistry. 2019;18(2):1950009.
- Vemula, P, D Dodda, U Balekari, S Panga and C Veeresham. “Simultaneous determination of Linagliptin and metformin by reverse phase-high performance liquid chromatography method: An application in quantitative analysis of pharmaceutical dosage forms.” Journal of Advanced Pharmaceutical Technology & Research. 2015;6(1):25-8.
- Yeoh, PH, KZ Lim, EL Tan, L Rhyman, Y Umar, HH Abdallah and P Ramasami. "Internal rotation of 2-, 3- and 4- Pyridine carboxaldehydes and their chalcogen analogues (S and Se) in the gas and solution phases: A theoretical investigation.” Journal of Solution Chemistry. 2016;45(8):1195-1212.

