PHYTOCHEMICAL ASSESSMENT, TOTAL PHENOLIC CONTENT, CYTOTOXIC, ANTIOXIDANT AND ANTIDIABETIC ACTIVITIES OF Hyoscyamus insanus

- Keywords: antidiabetic, antioxidant, cytotoxic, Hyoscyamus insanus
Copyright (c) 2021 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
Abstract
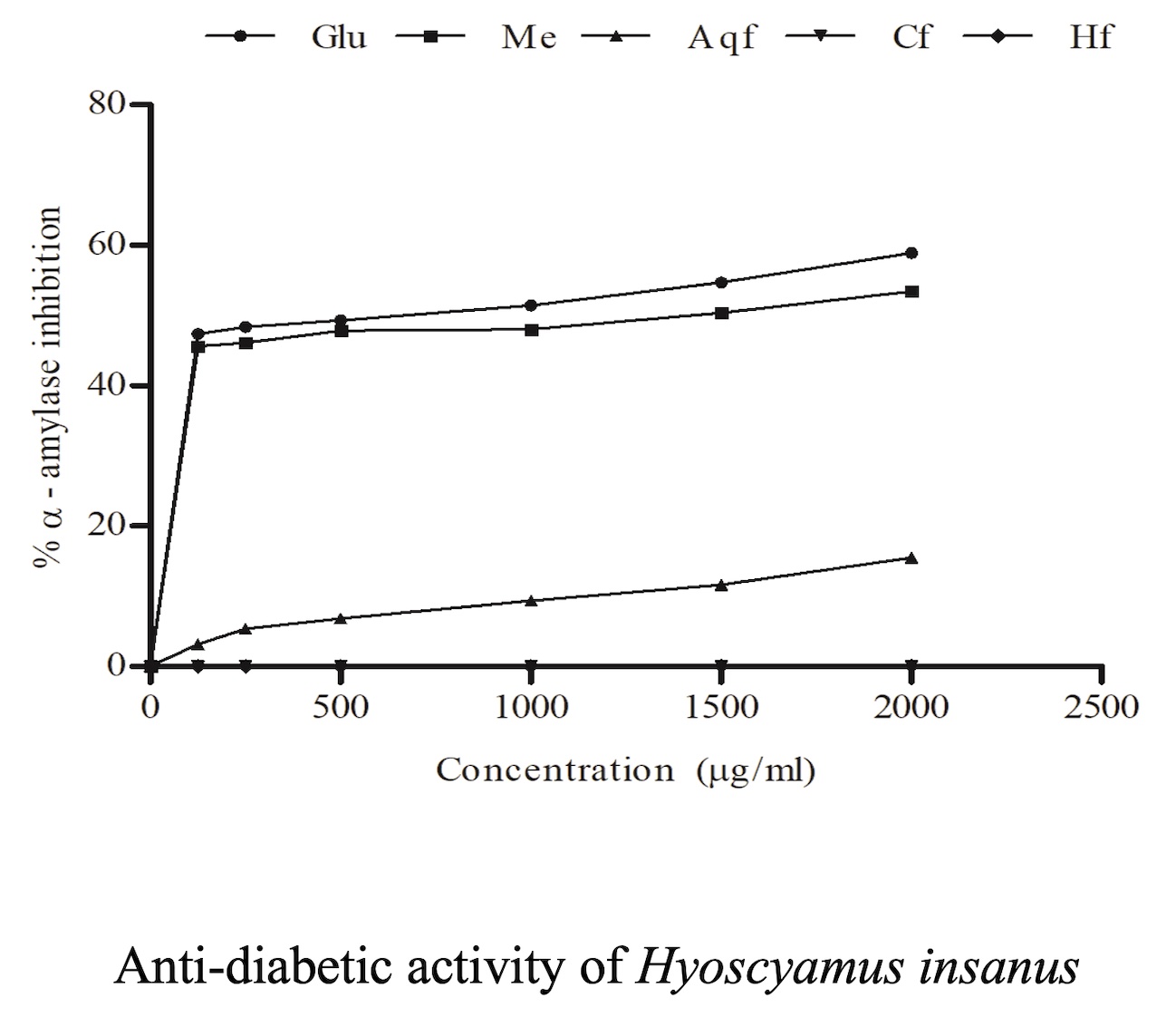
Hyoscyamus insanus (family Solanaceae) was traditionally used for treatment of asthma, relieving pain and increasing female body weight. The present projects was intended to assess the phytochemicals, total phenolic content, cytotoxic, antioxidant and antidiabetic activities of Hyoscyamus insanus leaves methanolic extract and its special fractions. After extraction, the methanolic extract was sequentially fractionated with hexane, chloroform and water followed by phytochemicals analysis of each sample. Brine shrimp lethality bioassay, Folin-Ciocalteu phenol reagent, DPPH, ABTS•+, H2O2 and α-amylase inhibition assay were employed for the assessment of cytotoxic property, total phenolic contents, antioxidant and anti-diabetic potency of methanolic extract and its fractions. Amino acids and protein, carbohydrates, flavonoids and saponins were found in the methanolic extract and aqueous fraction while chloroform fraction exhibited carbohydrates, flavonoids and saponins. The highest cytotoxic activity (80.6±1.2%) was exhibited by methanolic extract while maximum phenolic contents (21.93±1.17 mg GAE/g) were found in the chloroform fraction. The employed antioxidant assays expressed different results i.e. methanolic extract demonstrated highest 83.99%, 74.19% and 51.15% free radical scavenging characteristics in ABTS, DPPH and H2O2 assays respectively. The methanolic extract showed significant (53.44%) antidiabetic properties. The correlation of total phenolic contents with percentage antioxidant and anti-diabetic capabilities of methanolic extract and its special fractions was observed to be non-significant (P> 0.05). It is concluded that the Hyoscyamus insanus is a potential source of phenolic, cytotoxic, antioxidant and antidiabetic compounds.
References
- References
- Agarwal, A., Gupta, D., Yadav, G., Goyal, P., Singh, P. K., & Singh, U. 2009. An evaluation of the efficacy of licorice gargle for attenuating postoperative sore throat: a prospective, randomized, single-blind study. Anesthesia & Analgesia 109(1): 77-81
- Arnous, A., Makris, D. P., & Kefalas, P. 2001. Effect of principal polyphenolic components in relation to antioxidant characteristics of aged red wines. Journal of Agricultural and Food Chemistry 49(12): 5736-5742
- Branen, A. 1975. Toxicology and biochemistry of butylated hydroxyanisole and butylated hydroxytoluene. Journal of the American Oil Chemists’ Society 52(2): 59
- Cheng, A. Y., & Fantus, I. G. 2005. Oral antihyperglycemic therapy for type 2 diabetes mellitus. Canadian Medical Association Journal 172(2): 213-226
- Escarpa, A., & González, M. 2001. Approach to the content of total extractable phenolic compounds from different food samples by comparison of chromatographic and spectrophotometric methods. Analytica Chimica Acta 427(1): 119-127
- Fiore, C., Eisenhut, M., Ragazzi, E., Zanchin, G., & Armanini, D. 2005. A history of the therapeutic use of liquorice in Europe. Journal of ethnopharmacology 99(3): 317-324
- Funke, I., & Melzig, M. F. 2006. Traditionally used plants in diabetes therapy: phytotherapeutics as inhibitors of alpha-amylase activity. Revista Brasileira de Farmacognosia 16(1): 1-5
- Gyamfi, M. A., Yonamine, M., & Aniya, Y. 1999. Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally-induced liver injuries. General Pharmacology: The Vascular System 32(6): 661-667
- Hagerman, A. E., Riedl, K. M., Jones, G. A., Sovik, K. N., Ritchard, N. T., Hartzfeld, P. W., & Riechel, T. L. 1998. High molecular weight plant polyphenolics (tannins) as biological antioxidants. Journal of Agricultural and Food Chemistry 46(5): 1887-1892
- Hajrasouliha, S., Massoumi, A. A., TaherNejadSattar, S., Hamdi, M., & Mehregan, I. 2014. A phylogenetic analysis of Hyoscyamus L.(Solanaceae) species from Iran based on ITS and trnL-F sequence data. Jbes 5(1): 647-654
- Ismail, A., Marjan, Z. M., & Foong, C. W. 2004. Total antioxidant activity and phenolic content in selected vegetables. Food Chemistry 87(4): 581-586
- Kalpana, S., Vijai, D., & Premalatha, S. 2016. Antioxidant activity of different solvent extracts of Barleria longiflora Linn. International Journal of Current Research in Biology and Medicine 1(5): 1-8
- Karadag, A., Ozcelik, B., & Saner, S. 2009. Review of methods to determine antioxidant capacities. Food analytical methods 2(1): 41-60
- Katewa, S., Chaudhary, B., & Jain, A. 2004. Folk herbal medicines from tribal area of Rajasthan, India. Journal of ethnopharmacology 92(1): 41-46
- Kazazic, M., Djapo, M., & Ademovic, E. 2016. Antioxidant activity of water extracts of some medicinal plants from Herzegovina region. Int. J. Pure App. Biosci 4(2): 85-90
- Keawpradub, N., Dej-adisai, S., & Yuenyongsawad, S. (2005). Antioxidant and cytotoxic activities of Thai medicinal plants named Khaminkhruea: Arcangelisia flava, Coscinium blumeanum and Fibraurea tinctoria. Songklanakarin Journal of Science and Technol 27(2): 455-467
- Khan, D. 2008. Plant-size data and estimation of some vital leaf characteristics in naturally growing Nicotiana plumbaginifolia Viv.(Solanaceae) in Karachi. International Journal of Biolology & Biotech 5(1-2): 111-123
- Kumar, K. J. (2006). Effect of geographical variation on contents of tannic acid, gallic acid, chebulinic acid and ethyl gallate in Terminalia chebula fruits. Natural Products: An Indian Journal 2(3): 100-104
- KWON, Y. I., Apostolidis, E., & Shetty, K. 2007. Evaluation of pepper (Capsicum annuum) for management of diabetes and hypertension. Journal of Food Biochemistry 31(3): 370-385
- Losso, J. N., Shahidi, F., & Bagchi, D. 2007. Anti-angiogenic functional and medical foods: CRC Press.
- Malviya, N., Jain, S., & Malviya, S. 2010. Antidiabetic potential of medicinal plants. Acta Pol Pharm 67(2): 113-118
- Manikandan, R., Anand, A. V., & Kumar, S. 2016. Phytochemical and In vitro Antidiabetic Activity of Psidium Guajava Leaves. Pharmacognosy Journal 8(4):
- Meyer, B., Ferrigni, N., Putnam, J., Jacobsen, L., Nichols, D. j., & McLaughlin, J. L. 1982. Brine shrimp: a convenient general bioassay for active plant constituents. Planta medica 45(05): 31-34
- Miliauskas, G., Venskutonis, P., & Van Beek, T. 2004. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chemistry 85(2): 231-237
- Mukesh, R., & Namita, P. 2013. Medicinal plants with antidiabetic potential-a review. American-Eurasian Journal of Agriculture and Environmental Science 13(1): 81-94
- Nair, S. S., Kavrekar, V., & Mishra, A. 2013. In vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. European Journal of Experimental Biology 3(1): 128-132
- Ngoci, S. N., Mwendia, C. M., & Mwaniki, C.G. 2011. Phytochemical and cytotoxicity testing of Indigofera lupatana Baker F. Journal of Animal and Plant Sciences 1 (11): 1364-1373.
- Ogunwenmo, K. O., Idowu, O. A., Innocent, Chukwudi, E., Edward B., & Oyelana, O. A. 2007. Cultivars of Codiaeum variegatum (L.) Blume (Euphorbiaceae) show variability in phytochemical and cytological characteristics. African Journal of Biotechnology 6(20): 2400-2405
- Pal, R. S., Ariharasivakumar, G., Girhepunjhe, K., & Upadhay, A. 2009. In-vitro antioxidative activity of phenolic and flavonoid compounds extracted from seeds of Abrusprecatorius. International Journal Pharmaceutical Science 1(2): 136-140
- Pereira, J. A., Oliveira, I., Sousa, A., Ferreira, I. C., Bento, A., & Estevinho, L. 2008. Bioactive properties and chemical composition of six walnut (Juglans regia L.) cultivars. Food and chemical toxicology 46(6): 2103-2111
- Rates, S. M. K. 2001. Plants as source of drugs. Toxicon 39(5): 603-613
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free radical biology and medicine 26(9): 1231-1237
- Rice-Evans, C. A., Miller, N. J., & Paganga, G. (996. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free radical biology and medicine 20(7): 933-956
- Sahreen, S., Khan, M. R., & Khan, R. A. 2017. Evaluation of antioxidant profile of various solvent extracts of Carissa opaca leaves: an edible plant. Chemistry Central Journal 11(1): 83
- Shafi, S., & Tabassum, N. 2013. Survey on Anti-Diabetic Plants in Kashmir [India]. Journal of Advanced Pharmacy Education & Research 3(4): 306-318
- Sharifi, G., Kouhsari, S., Ebrahimzadeh, H., & Khatamsaz, M. 2006. Isozyme analysis of seedling samples in some species of Hyoscyamus from Iran. Pakistan Journal of Biological Sciences 9(9): 1685-1692
- Sheidai, M., Mosallanejad, M., & Khatamsaz, M. (1999). Karyological studies in Hyoscyamus species of Iran. Nordic journal of botany 19(3): 369-374
- Shibata, S. (1994). Antitumor-promoting and anti-inflammatory activities of licorice principles and their modified compounds: ACS Publications.
- Singleton, V., & Rossi, J. A. 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American journal of Enology and Viticulture 16(3): 144-158
- Singleton, V. L., Orthofer, R., & Lamuela-Raventós, R. M. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in enzymology 299: 152-178
- Tafaghodi, M., & Rahimizadeh, M. 2003. Isolation and identification of Hyoscyamus insanus alkaloids. Journal of Medicinal Plants 3(7): 29-36
- Trease, G., & Evans, W. 1989. Phytochemical screening. Pharmacognsy. 11th edn. Brailliar Tiridel Can: Macmillian Publishers, London, England.
- Tupe, R., Kemse, N., & Khaire, A. 2013. Evaluation of antioxidant potentials and total phenolic contents of selected Indian herbs powder extracts. International Food Research Journal 20(3): 1053- 1063
- Umashanker, M., & Shruti, S. 2011. Traditional Indian herbal medicine used as antipyretic, antiulcer, anti-diabetic and anticancer: A review. International journal of research in pharmacy and chemistry, 1(4), 1152-1159
- Vasu, P., Khan, N. D., Khan, Z. H., & Mular, S. 2017. In vitro antidiabetic activity of methanolic extract of Citrus limon, Punica granatum, Musa acuminata peel. International Journal of Applied Research 3(4): 804-806
- Wong, S. P., Leong, L. P., & Koh, J. H. W. 2006. Antioxidant activities of aqueous extracts of selected plants. Food Chemistry 99(4): 775-783
- Zhang, Z., Liao, L., Moore, J., Wu, T., & Wang, Z. 2009. Antioxidant phenolic compounds from walnut kernels (Juglans regia L.). Food Chemistry 113(1): 160-165

