CHROMIUM (III) COMPLEX OF 2-AMINO-3-CARBOMETHOXY-4,5,6,7-TETRAHYDROBENZO[B] THIOPHENE: SYNTHESIS, CHARACTERIZATION AND ANTIMICROBIAL ACTIVITY

- Antimicrobial, Complexation reaction, 4,5,6,7-tetrahydrobenzo[b]- thiophene, Chromium complex
Copyright (c) 2020 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
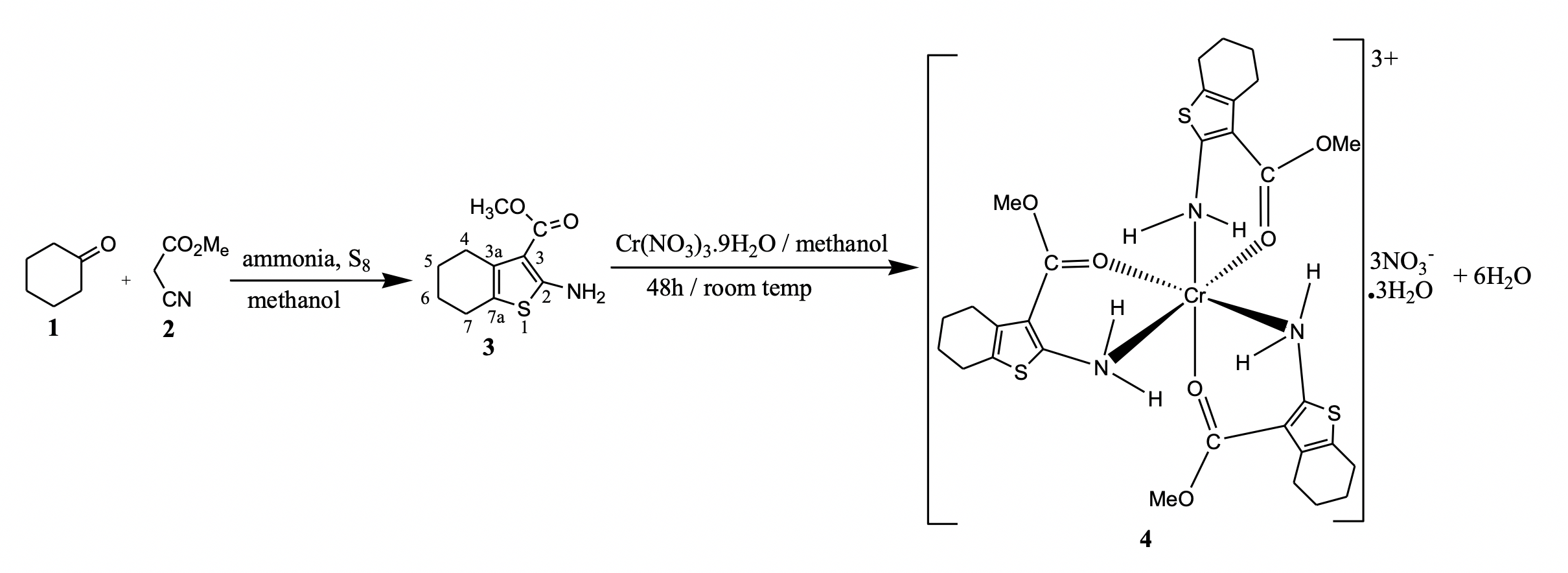
A new chromium (III) coordination compound of 2-amino-3-carbomethoxy-4,5,6,7-tetrahydrobenzo[b]thiophene (ACTT) (3) with Cr(NO3)3.9H2O in methanol at room temperature for 48 hours has been prepared. The composition of the new complex compound (4) has been confirmed by a number of instrumental methods including IR, NMR, MS and elemental analysis. It was found that the ACTT ligand behaves as bidentate chelating agent and coordinates to the central metal ion through the nitrogen atom of the amino-group and the oxygen atom of the carbonyl function of the ester group. The results showed that in the coordination sphere of the complex, the metal ion is coordinated by three chelating ACTT ligands. In the structure of the cation core [Cr(ACTT)3]3+, the three ACTT ligands are linked to the chromium ion through three Cr-N and three Cr-O bonds forming a square bipyramidal geometry. The metal complex and the ACTT ligand were screened for their antimicrobial activities against several strains of bacteria (Staphylococcus aureus ATCC25923, Escherichia coli S2 (1), Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa ATCC27853, Shigella flexneri SDINT) and fungi (Candida albicans ATCC10231, Candida tropicalis PK233, Cryptococcus neoformans H99). The chromium complex showed promising antimicrobial activity against the microorganisms with minimum inhibitory values between 4 and 16 μg/mL.
References
- K. Aruna, S. Z. Bootwala, M. Tariq, C. Fernandes, Int. J. Pharm. Biological Sci. 5, 440, (2014)
- S. Kumar, N. Kumar, Int. Curr. Pharm. J. 2, 91, (2013)
- M. Bouachrine, O. Benaqqa, H. Toufik, M. Hamidi, J. - P. Lère-Porte, F. Serein-Spirau, A. Amine, Analele Universităţii din Bucur. 19, 44, (2010)
- R. R. Zaky, A. M. Abdelghay, Res. J. Pharm. Biological Chem. Sci. 2, 764, (2011)
- R. M. Mohareb, A. A. Fahmy, Eur. Chem. Bull. 2, 553, (2013)
- A. Singh, S. P. Singh, A. Amalraj, P. D. Gokulan, P. Porwal, Int. J. Pharm. Biological Arch. 2, 540, (2011)
- W. W. Wardakhana, N. A. Loucab, M. M. Kamel, Acta Chim. Slov. 54, 241, (2007)
- V. Alagarsamy, D. Shankar, V. R. Solomon, Arkivoc xvi, 159, (2006)
- B. Narayana, B. V. Ashalatha, K. K. V. Raj, N. S. Kumari, Indian J. Chem. 45B, 2703, (2006)
- M. A. Gouda, H. F. Eldien, M. M. Girges, M. A. Berghot, Med. Chem. 3, 232, (2013)
- S. Turk, M. Hrast, I. Sosi, H. Barreteau, D. Mengin-Lecreulx, D. Blanot, S. Gobec, Acta Chim. Slov. 60, 299, (2013)
- K. - T. Wong, R. - T. Chen, Tetrahedron Lett. 43, 3317, (2002)
- S. A. Khan, A. Y. Obaid, L. M. Al-Harb, M. N. Arshad, A. M. Asiri, M. B. Hursthouse, Int. J. Electrochem. Sci. 10, 2323, (2015)
- N. R. Patil, R. M. Melavanki, H. D. Patil, D. Nagaraja, F. M. Sanningannavar, Int. J. Life Sci. Pharm. Res. 3, 76, (2013)
- T. V. Dubinina, D. V. Dyumaeva, S. A. Trashin, M. V. Sedova, A. B. Karpo, V. I. Krasovskii, L. G. Tomilova, Macroheterocycles 5, 156, (2012)
- F. A. Mohamed, A. E. El-Alfy, J. Appl. Sci. Res. 9, 183, (2013)
- H. R. Maradiya, Turk. J. Chem. 25, 450, (2001)
- Md. A. Hossain, Md. C. Sheikh, S. K. A. Z. Mahmud, Md. A. Alam, Int. J. Sci. Tech. Res. 2, 237, (2013)
- R. M. S. Pereira, N. E. D. Andrades, N. Paulino, A. C. H. F. Sawaya, M. N. Eberlin, M. C. Marcucci, G. M. Favero, E. M. Novak, S. P. Bydlowski, Molecules 12, 1366, (2007)
- A. D. Bansod, R. G. Mahale, A. S. Aswar, J. Indian Chem. Soc. 83, 781, (2006)
- R. A. Yaul, V. V. Dhande, B. G. Pethe, A. S. Aswar, Bull. Chem. Soc. Ethiop. 28, 264, (2014)
- V. B. Devi, P. Arulmozhichelvan, P. Murugakoothan, Int. J. ChemTech Res. 6, 2140, (2014)
- A. A. Alemi, B. Shaabani, Acta Chim. Slov. 47, 369, (2000)
- C. - C. Ko, W. - W. V. Yam, J. Mater. Chem. 20, 2070, (2010)
- T. Yutaka, I. Mori, M. Kurihara, J. Mizutani, K. Kubo, S. Furusho, K. Matsumura, N. Tamai, H. Nishihara, Inorg. Chem. 40, 4995, (2001)
- A. Samadi-Maybodi, S. K. H. Nejad-Darzi, R. Akhoondi, Int. Nano Lett. 1, 58, (2011)
- M. Rizzotto in Search for Antibacterial Agents, A. V. Bobbarala ed. InTech, Croatia, 2012; pp. 80-81.
- H. Al-Talib, A. Al-Khateeb, H. Hassan, Int. Med. J. 22, 3, (2015)
- T. J. Foster, Fed. Eur. Microbiol. Soc. 41, 449, (2017)
- M. Goncuoglu, F. S. B. Ormancı, N. D. Ayaz, I. Erol Ann. Microbiol. 60, 494, (2010)
- C. Kim, M. Fulke, A. Rahemi, T. Taghavi, A. Asmare, P. Kaseloo, E. Ndegwa, E. Sismour, EC Nutrition 13, 52, (2018)
- A. - M. Guérout-Fleury, K. Shazand, N. Frandsen, P. Stragier, Gene 167, 336, (1995)
- R. E. W. Hancock, D. P. Speert, Drug Resist. Updates 3, 255, (2000)
- P. A. Lambert, J. R. Soc. Med. 95, 26, (2002)
- S. Mehata, G. Duan, W. Zhang, Int. J. Infect. Microbiol. 1, 48, (2012)
- A. A. M. Lima, J. J. C. Sidrim, N. L. Lima, W. Titlow, M. E. Evans, R. N. Greenberg, J. Clin. Microbiol. 35, 1065, (1997)
- M. Sanguinetti, B. Posteraro, C. Lass-Flörl, Mycoses 58, 13, (2015)
- M. Maroszyńska, A. Kunicka-Styczyńska, K. Rajkowska, I. Maroszyńska, Acta Biochim. Pol. 60, 724, (2013)
- R. J. Kothavade, M. M. Kura, A. G. Valand, M. H. Panthak, J. Med. Microbiol. 59, 880, (2010)
- J. Santhanam, A. Fairuz, F. K. Ooi, A. H. Muhamad, J. Kesihat. Masy. Isu Khas 15, 53, (2002)
- M. A. Maligie, C. P. Selitrennikoff, Agents Chemother. 49, 2856, (2005)
- M. Lozano-Chiu, V. L. Paetznick, M. A. Ghannoum, J. H. Rex, J. Clin. Microbiol. 36, 2822, (1998)
- E. S. Fondjo, D. A. Siéwé, J. - D. - D. Tamokou, S. E. Ekom, S. K. D. Djeukoua, G. Doungmo, M. E. Walters, A. Tsopmo, P. F. W. Simon, J. R. Kuiate, Acta Chim. Slov. 67, 211, (2020)
- M. G. Reinecke, T. A. Woodrow, E. S. Brown, J. Org. Chem. 57, 1021, (1992)
- J. D. Tamokou, M. F. Tala, H. K. Wabo, J. - R. Kuiate, P. Tane, J. Ethnopharmacol. 124, 575, (2009)
- S. S. Patil, M. M. Shaikh, Acta Pol. Pharm. Drug Res. 69, 686, (2012)
- K. Nakamoto, Infrared and Raman spectra of inorganic and coordination compounds; Part B: Applications in coordination, organometallic, and bioinorganic chemistry, John Wiley & Sons, New Jersey, 2009.
- E. J. House, Inorganic Chemistry, Elsevier, San Diego, 2008.
- K. K. Ilavenil, M. Dhamodharan, J. Chem. Pharm. Sci. 9, 1462, (2016)
- C. Zhu, Y. Wang, Q. Mao, F. Li, Y. Li, C. Chen, Materials 313, 13, (2017)
- A. Debnath, F. Hussain, D. T. Masram, Bioinorg. Chem. Appl. 2014, 8, (2014)
- V. Balamurugan, S. Sankar, Int. J. Res. Dev. Pharm. Life Sci. 4, 1327, (2015)
- R. Al-Hassany, A. T. Mahmood, E. Z. Mohamed, A. A. Baqimaryoosh, A. A. R. Hussein, Acta Chim. Pharm. Indica 6, 25, (2016)
- P. Jain, V. Singh, Der Pharm. Chem. 8, 180, (2016)
- M. G. Djouossi, J. - D. Tamokou, D. Ngnokam, J. - R. Kuiate, L. A. Tapondjou, D. Harakat, L. Voutquenne-Nazabadioko, BMC Complementary Altern. Med. 15, 8, (2015)
- J. S. Chandra, Y. A. S. J. P. Kumaria, P. N. V. V. L. P. Rania, Y. Sunandamma, Indian J. Adv. Chem. Sci. 2, 37, (2013)
- M. Thankamony, K. Mohanan, J. Serb. Chem. Soc. 46A, 251, (2007)

