- antibacterial,
- antibiotic resistance,
- levofloxacin,
- silver triflate,
- triflate
Copyright (c) 2020 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
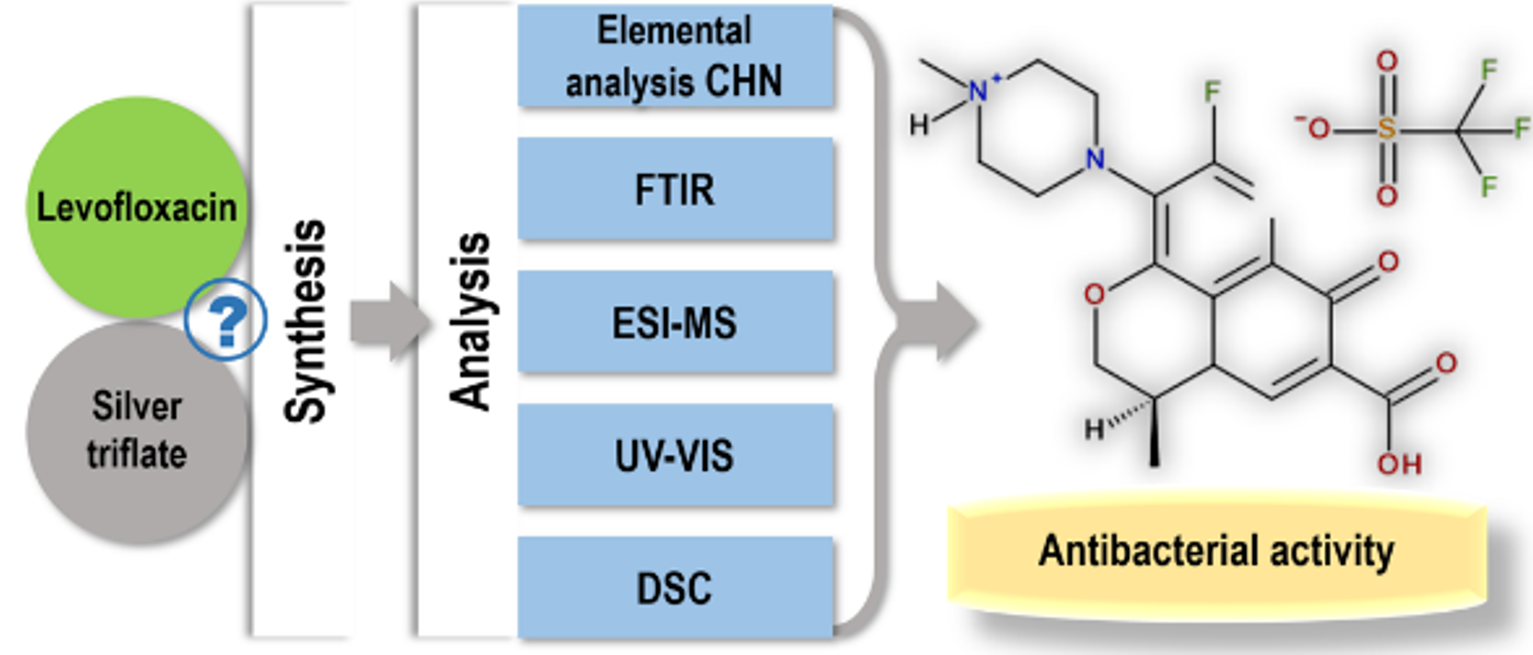
A new levofloxacin derivative using silver triflate with antibacterial activity was synthesized and characterized. The new compound has been physicochemically characterized through elemental analysis, spectroscopic and thermal methods. All correlated experimental data suggested that the levofloxacin triflate was obtained. The antibacterial activity of the new compound was tested against six Gram-positive and Gram-negative bacteria. In vitro, the new compound had similar activity to levofloxacin against Staphylococcus aureus, Escherichia coli and Klebsiella pneumoniae and very closed to the minimum inhibitory concentration values of levofloxacin against Staphylococcus aureus MRSA, Enterococcus faecalis, and Pseudomonas aeruginosa.
References
- S.E. Edwards, C.M. Morel, Expert. Rev. Pharmacoecon. Outcomes Res. 19, 685, (2019)
- D. Buckland, Prescriber. 28, 12, (2017)
- Tuberculosis. 88, 2, (2008)
- G.J. Noel, Clinical Medicine. Therapeutics. 1, 433, (2009)
- A.M. Noreddin, W.F. Elkhatib, K.M. Cunnion and G.G. Zhanel, Drug. Healthc. Patient Saf. 3, 59, (2011)
- L.S. Redgrave, S.B. Sutton, M.A. Webber, L.J. Piddock, Trends Microbiol. 22, 438, (2014)
- G.A.R.Y. Suaifan, A.A.M. Mohammed, Bioorganic & Medicinal Chemistry. 27, 3005, (2019)
- H.A.A. Ezelarab, S.H. Abbas, H.A. Hassan, G.E.-D.A. Abuo-Rahma, Arch. Pharm. (Weinheim). 351, e1800141, (2018)
- A. Rusu, G. Hancu, G. Tóth, S. Vancea, F. Toma, A.D. Mare, A. Man, G.M. Niţulescu, V. Uivarosi, J. Mol. Struct. 1123, 384, (2016)
- Clinical and Laboratory Standards Institute, M100-S23 Performance Standards for Antimicrobial Susceptibility Testing Twenty-Third Informational Supplement, Wayne, PA, USA: Clinical and Laboratory Standards Institute, 2013.
- A. Rusu, G. Hancu, F. Toma, A.D. Mare, A. Man, B.S. Velescu, V. Uivarosi, Farmacia. 64, 922, (2016)
- B. Quillian, A.E. Fields, D. Chace, A.M. Vickery, M. Sharma, D. Zurwell, J.G. Bazemore, L. Phan, D. Thomas, C.W. Padgett, Inorg. Chim. Acta. 489, 224, (2019)
- W.-C. Pan, M.-M. Zhang, J.-Q. Liu, X.-S. Wang, Syntesis. 51, 3101, (2019)
- P.J. Malinowski, Z. Mazej, M. Derzsi, Z. Jagličić, J. Szydłowska, T. Gilewski, W. Grochala, CrystEngComm. 13, 6871, (2011)
- V.L. Dorofeev, A.P. Arzamastsev, O.M. Veselova, Pharm. Chem. J. 38, 333, (2004)
- PubChem Database, National Center for Biotechnology Information. https://pubchem.ncbi.nlm.nih.gov/compound/Trifluoromethanesulfonic-acid. [Accessed 27 05 2020]
- Sigma Aldrich Catalog, Merck KgaA. https://www.sigmaaldrich.com/catalog/product/aldrich/176435?lang=en®ion=RO [Accessed 27 05 2020]
- W. Geary, Coord. Chem. Rev. 7, 81, (1971)
- I. Ali, W.A. Wani, K. Saleem, Synth. React. Inorg., Met.-Org., Nano-Met. Chem. 43, 1162, (2013)
- V.L. Dorofeev, Pharm. Chem. J. 38, 693, (2004)
- P.C. Huber, G.P. Reis, M.C.K. Amstalden, M. Lancellotti, W. P. Almeida, Polyhedron. 57, 14, (2013)
- H.-R. Park, T. H. Kim, K.-M. Bark, Eur. J. Med. Chem. 37, 443, (2002)
- D. H. Johnston, D. F. Shriver, Inorganic Chemistry. 32, 1045, (1993)
- P. Larkin. IR and Raman Spectroscopy Principles and Spectral Interpretation, Elservier, Amsterdam, 2011.
- M. Refat, Spectrochim. Acta A Mol. Biomol. Spectrosc. 68, 1393, (2007)
- J. Coates in Interpretation of infrared spectra, a practical approach, R.A. Meyers, ed. John Wiley& Sons Ltd., Chichester, 2000 ; pp. 10815
- A.S. Sadeek, J. Mol. Struct. 753, 1 (2005)
- I. Sousa, V. Claro, J.L. Pereira, A.L. Amaral, L. Cunha-Silva, B.d. Castro, M.J. Feio, E. Pereira, P. Gameiro, J. Inorg. Biochem. 110, 64, (2012)
- K. Nakamuto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, Wiley, Hoboken, 2009
- M.J. O'Neil in An Encyclopedia of Chemicals, Drugs, and Biologicals, M.J. O’Neil, P.E. Heckelman, C.B. Koch, K.J. Roman eds. John Wiley & Sons, Hoboken, 2006 ; pp. 1171.
- E.D. Márquez, E.V. Santiago, S.H. López, Physical Chemistry. 9, 1, (2019)