ANTI-OXIDANT AND ANTI-INFLAMMATORY POTENTIAL OF SECONDARY METABOLITES FROM Daphne mucronata ROYLE AND THEIR FIRST-PRINCIPLES INVESTIGATIONS

- Daphne mucronata Royle,
- Mucronin-C,
- Antioxidant activity,
- Anti-inflammatory activity,
- Density functional theory
Copyright (c) 2021 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
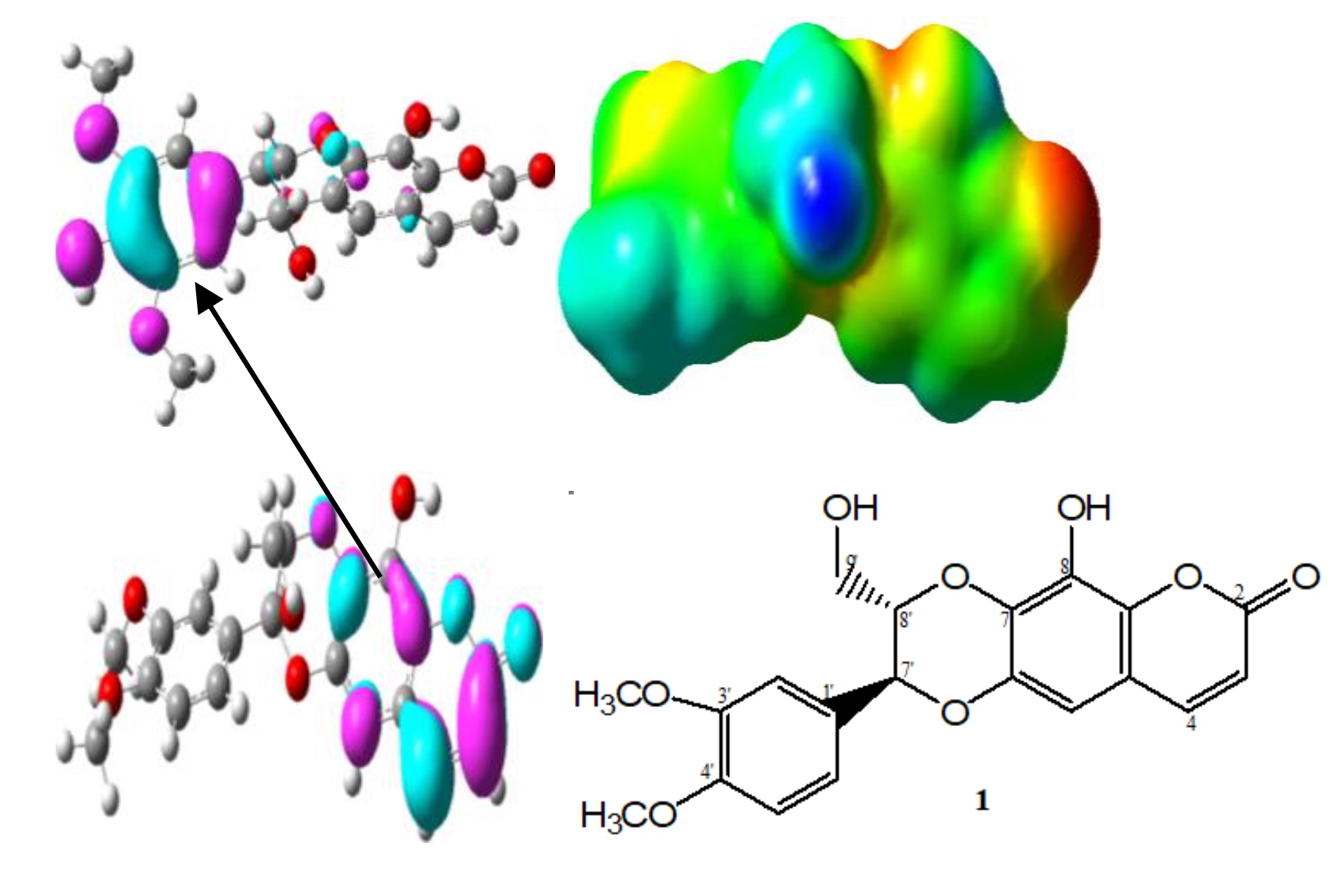
Ten coumarin class of compounds, including new fused coumarinolignoids namely, mucronin-C (1) were isolated from the methanol extract from whole plant of Daphne mucronata Royle. The structures of mucronin-C (1) and its configurations were determined by chemical and spectroscopic methods including 1DNMR, 2D-NMR and HR-FAB-MS. The isolated compounds 1-10 were evaluated for in-vitro biological activities. The anti-inflammatory (lipoxygenase) activities of compound 1, 4, 8, 9 and 10 (IC50 = 21.7, 23.7, 25.1, 27.3 and 26.0 µg/mL, respectively) were higher compared with standard Quercetin (IC50 = 22.5 µg/mL). The antioxidant property of coumarin as evaluated by DPPH scavenging bioassay was significantly greater in compounds 1-5, 9 and 10 (IC50 = 0.7, 0.8, 1.9, 2.3, 2.8, 0.5 and 2.1 µg/mL respectively) as standard Trolox (IC50 = 0.3 µg/mL). The density functional descriptors in the progress of quantitative structure-activity relationship (QSAR) are significant to analyze the reactive sites and antioxidant ability of compounds. We have explored the reactive sites and radical scavenging activity of studied coumarin derivatives by shedding light on electron affinity, ionization potential, molecular electrostatic potential, frontier molecular orbitals and molecular descriptors analysis. First-principles calculations about one-electron transfer mechanism revealed that smaller ionization potential value of Compounds 1-5, 9 and 10 are leading to superior antioxidant activity, which is in good agreement with the experimental data.
References
- REFERENCES
- S. Oran , D. Al-Eisawi, Check-list of medicinal plants in Jordan. Dirasat 25(2) 84-112 (1998).
- J.-J. Chen, T.-Y. Wang , T.-L. Hwang, Neolignans, a coumarinolignan, lignan derivatives, and a chromene: anti-inflammatory constituents from Zanthoxylum avicennae. Journal of natural products 71(2) 212-217 (2008).
- K. N. Venugopala, V. Rashmi , B. Odhav, Review on natural coumarin lead compounds for their pharmacological activity. BioMed research international 2013 (2013).
- M. A. Rasool, M. Imran, H. Nawaz, A. Malik , S. U. Kazmi, Phytochemical studies on Daphne mucronata. Journal-Chemical Society of Pakistan 31(5) 845-850 (2009).
- Z. Khodadadian, M. Hassanpour-Ezatti, S. Z. Mousavi , J. Asgarpanah, Analgesic and anti-inflammatory potential of aerial parts of the Daphne mucronata Royle extract in mice: Opioid-independent action. Asian Pacific Journal of Tropical Biomedicine 6(3) 198-201 (2016).
- N. Ullah, S. Ahmed, P. Muhammad, Z. Ahmed, H. R. Nawaz , A. Malik, Coumarinolignoid glycoside from Daphne oleoides. Phytochemistry 51(1) 103-105 (1999).
- S. Ferheen, M. A. Rasool, B. Naqvi, M. Imran, R. A. Khan, M. A. Kalhoro , A. Malik, Anti-tuberculosis coumarinolignans from Daphne mucronata. Asian Journal of Chemistry 26(21) 7262 (2014).
- S. Collenette, Wildflowers of Saudi Arabia1999: National Commission for Wildlife Conservation and Development (NCWCD).
- M. Ballero, F. Poli, G. Sacchetti , M. C. Loi, Ethnobotanical research in the territory of Fluminimaggiore (south-western Sardinia). Fitoterapia 72(7) 788-801 (2001).
- W. Murad, A. Ahmad, S. A. Gilani , M. A. Khan, Indigenous knowledge and folk use of medicinal plants by the tribal communities of Hazar Nao Forest, Malakand District, North Pakistan. Journal of Medicinal Plants Research 5(7) 1072-1086 (2011).
- M. A. Abdelgawad, M. B. Labib, W. A. Ali, G. Kamel, A. A. Azouz , E.-N. EL-Shaymaa, Design, synthesis, analgesic, anti-inflammatory activity of novel pyrazolones possessing aminosulfonyl pharmacophore as inhibitors of COX-2/5-LOX enzymes: Histopathological and docking studies. Bioorganic chemistry 78 103-114 (2018).
- H. G. Choi, P. T. Tran, J.-H. Lee, B. S. Min , J. A. Kim, Anti-inflammatory activity of caffeic acid derivatives isolated from the roots of Salvia miltiorrhiza Bunge. Archives of pharmacal research 41(1) 64-70 (2018).
- R. Mogana, K. Teng-Jin , C. Wiart, Anti-inflammatory, anticholinesterase, and antioxidant potential of scopoletin isolated from Canarium patentinervium Miq.(Burseraceae Kunth). Evidence-based complementary and alternative medicine 2013 (2013).
- M. Allegra, Antioxidant and Anti-Inflammatory Properties of Plants Extract, 2019, Multidisciplinary Digital Publishing Institute.
- Y. J. Lee, W. I. Kim, S. Y. Kim, S. W. Cho, H. S. Nam, S. H. Lee , M. K. Cho, Flavonoid morin inhibits proliferation and induces apoptosis of melanoma cells by regulating reactive oxygen species, Sp1 and Mcl-1. Archives of pharmacal research 42(6) 531-542 (2019).
- E. Zielińska, B. Baraniak , M. Karaś, Identification of antioxidant and anti‐inflammatory peptides obtained by simulated gastrointestinal digestion of three edible insects species (Gryllodes sigillatus, Tenebrio molitor, Schistocerca gragaria). International journal of food science & technology 53(11) 2542-2551 (2018).
- E. Ahmed, M. Imran, A. Malik , M. Ashraf, Antioxidant activity with flavonoidal constituents fromAerva persica. Archives of pharmacal research 29(5) 343-347 (2006).
- C. Deby , G. Magotteaux, Relationship between essential fatty acids and tissue antioxidant levels in mice. Comptes Rendus des Seances de la Societe de Biologie et de ses Filiales 164(12) 2675-2681 (1970).
- S. Baylac , P. Racine, Inhibition of 5-lipoxygenase by essential oils and other natural fragrant extracts. International Journal of Aromatherapy 13(2-3) 138-142 (2003).
- L. L. Mensor, F. S. Menezes, G. G. Leitão, A. S. Reis, T. C. d. Santos, C. S. Coube , S. G. Leitão, Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytotherapy research 15(2) 127-130 (2001).
- M. Najafi , S. A. R. Naqvi, Theoretical study of the substituent effect on the hydrogen atom transfer mechanism of the irigenin derivatives antioxidant action. Journal of Theoretical and Computational Chemistry 13(02) 1450010 (2014).
- D. Mikulski, K. Eder , M. Molski, Quantum-chemical study on relationship between structure and antioxidant properties of hepatoprotective compounds occurring in Cynara scolymus and Silybum marianum. Journal of Theoretical and Computational Chemistry 13(01) 1450004 (2014).
- K. SADASIVAM, R. JAYAPRAKASAM , R. KUMARESAN, A DFT STUDY ON THE ROLE OF DIFFERENT OH GROUPS IN THE RADICAL SCAVENGING PROCESS. Journal of Theoretical and Computational Chemistry 11(04) 871-893 (2012).
- A. Mahmood , A. Irfan, First principles investigations of geometric, electronic and optical properties of 5-aminotetrazole derivatives. Journal of Computational Electronics 12(3) 437-447 (2013).
- A. Irfan, M. Assiri , A. G. Al-Sehemi, Exploring the optoelectronic and charge transfer performance of diaza[5]helicenes at molecular and bulk level. Organic Electronics 57 211-220 (2018).
- J. Preat, D. Jacquemin , E. A. Perpète, Design of New Triphenylamine-Sensitized Solar Cells: A Theoretical Approach. Environmental Science & Technology 44(14) 5666-5671 (2010).
- A. G. Al-Sehemi , A. Irfan, Effect of donor and acceptor groups on radical scavenging activity of phenol by density functional theory. Arabian Journal of Chemistry 10(Supplement 2) S1703-S1710 (2017).
- A. G. Al-Sehemi, A. Irfan, S. M. Aljubiri , K. H. Shaker, Density functional theory investigations of radical scavenging activity of 3′-Methyl-quercetin. Journal of Saudi Chemical Society 20, Supplement 1 S21-S28 (2016).
- A. G. Al-Sehemi, A. Irfan, S. M. Aljubiri , K. H. Shaker, COMBINED EXPERIMENTAL AND COMPUTATIONAL STUDY OF THE RADICAL SCAVENGING ACTIVITY OF LUTEOLIN. Journal of Theoretical and Computational Chemistry 12(05) 1350021 (2013).
- Frisch MJ, Trucks GW, Schlegel HB et al.,, Gaussian-16, Revision A.1, Gaussian, Inc., Wallingford, CT, 2016.
- B. Sajeli, M. Sahai, R. Suessmuth, T. Asai, N. Hara , Y. Fujimoto, Hyosgerin, a new optically active coumarinolignan, from the seeds of Hyoscyamus niger. Chemical and pharmaceutical bulletin 54(4) 538-541 (2006).
- A. Magalhães, M. D. G. Zoghbi , A. C. Siani, 5-Methoxypropacin, a novel coumarinolignoid from Protium unifoliolatum. Natural product research 20(1) 43-46 (2006).
- C. Ma, H. J. Zhang, G. T. Tan, N. V. Hung, N. M. Cuong, D. D. Soejarto , H. H. Fong, Antimalarial compounds from Grewia bilamellata. Journal of natural products 69(3) 346-350 (2006).
- P. Srivastava, V. K. Vyas, B. Variya, P. Patel, G. Qureshi , M. Ghate, Synthesis, anti-inflammatory, analgesic, 5-lipoxygenase (5-LOX) inhibition activities, and molecular docking study of 7-substituted coumarin derivatives. Bioorganic chemistry 67 130-138 (2016).
- A. Vektariene, G. Vektaris , J. Svoboda, A theoretical approach to the nucleophilic behavior of benzofused thieno [3, 2-b] furans using DFT and HF based reactivity descriptors. Arkivoc 7 311-329 (2009).
- P. Geerlings, F. De Proft , W. Langenaeker, Conceptual density functional theory. Chemical Reviews 103(5) 1793-1874 (2003).

