
- Band gap,
- carcinogenic azo dye Disperse Red 176.1,
- Cu2O,
- Equilibrium Isotherms and Kinetics,
- Photocatalytic degradation
Copyright (c) 2020 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
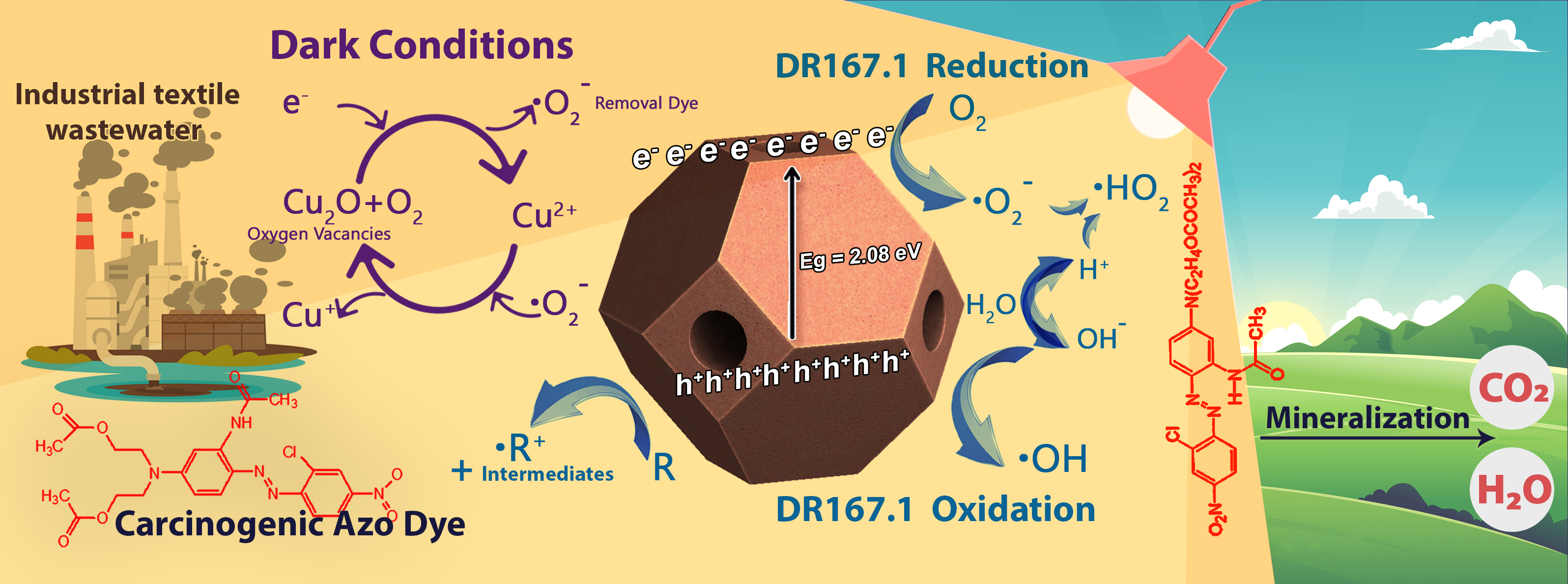
Refining and disposal of wastewater is an important concern of textile industries due to the presence of carcinogenic azo dyes. The monoazo dye Disperse Red 167.1 (DR167.1) is used in dyeing polyester and cotton fibers. In this study, Cage Cu2O particles was synthesized by using non-toxic and inexpensive materials as a bi-functional material that can mineralize this pollutant under light irradiation and dark media. The samples were characterized by FESEM, XRD, BET / BJH, FTIR, and DRS techniques. The crystallite size was almost 24.3 nm by the Williamson-Hall. Direct band gap energy was equal to 2.08 eV by Kubelka-Munk. It revealed a greater photocatalytic performance in visible light than UV-C and it absorbed 49% of the dye within 10 minutes in the darkness. The oxidation results indicated that at pH equal to 6.44 without the presence of H2O2, 0.75 mg.L-1 of cage Cu2O and of 94 mg.L-1 dye concentration yielded 91 % removal and mineralization during 10 min. Removal under the visible light irradiation increased to 93% upon increasing the time to 40 min. as dye concentration rose from 94 to 340 mg.L-1, with a slight reduction in the efficiency of the photocatalytic degradation process, the efficiency was obtained as 91%. Koble Corrigan model was found as the best isotherm model. Reaction kinetics followed the pseudo-second order model. Overall, due to its absorption and removal of dyes in a short time, it can be a good candidate for textile wastewater treatment.
References
- D. Zhang. Acta Chimica Slovaca 6(1), 141-149, ( 2013). DOI: 10.2478/acs-2013-0022
- Z. Zeng, Y.Yan, , J. Chen, , P. Zan, , Q. Tian, P. Chen. Adv. Funct. Mater, 29(2), 1806500,
- ( 2019). DOI: 10.1002/adfm.201806500
- Mohamed. R. M, Aazam. E. S. Appl Catal A Gen, 480, 100-107,(2014). DOI: 10.1016/j.apcata.2014.04.039
- H. D. Aghdam, H. Azadi, M. Esmaeilzadeh, S. M. Bellah, and R. Malekfar. Optical Materials, 91, 433-438, ( 2019). DOI: 10.1016/j.optmat.2019.03.027
- G.R. Surikanti, A.K. Bandarapu, M.V. Sunkara, ChemistrySelect, 4(8), 2249-2257,(2019). DOI: 10.1002/slct.201900003
- G. Wang, R. van den Berg, C. de Mello Donega, K.P. de Jong, P.E. de Jongh, P. E. Appl Catal,B, 192, 199-207, (2016). DOI: 10.1021/jp111778g
- Y. Jiang, H.Yuan, H. Chen, ChemPhysChem, 17(1), 630-637,(2015). DOI: 10.1039/C4CP03631J
- M. Iqbal, Y. Wang, H. Hu, M. He, A.H. Shah, L. Lin, P. Li, K. Shao, A.R. Woldu, T. He. Appl Surf Sci, 443, 209-216,(2018). DOI: 10.1016/j.apsusc.2018.02.162
- B. White, M. Yin, A. Hall, D. Le, S. Stolbov, T. Rahman, N. Turro, S. O'Brien. Nano let, 6(9), 2095-2098, (2006). DOI: 10.1021/nl061457v
- B. Wang, W. Zhang, Z. Zhang, R. Li, Y. Wu, Z. Hu, X. Wu, C. Guo, G. Cheng, R. Zheng. RSC advances, 6(105), 103700-103706,( 2016). DOI: 10.1039/C6RA22474A
- Q. Guo, Y. Li, W. Zeng. Physica E Low Dimens Syst Nanostruct, 114, 113564,(2019). DOI: 10.1016/j.physe.2019.113564
- R. Ji, W. Sun, Y. Chu. ChemPhysChem, 14(17), 3971-3976,(2013). DOI: 10.1002/cphc.201300735
- T. Aditya, J. Jana, N.K. Singh, A. Pal, T. Pal. ACS omega, 2(5), 1968-1984, (2017). DOI: 10.1021/acsomega.6b00447
- C.H. Kuo, C.H. Chen, M.H. Huang. Adv Funct Mater, 17(18), 3773-3780,(2007). DOI: 10.1002/adfm.200700356
- F. Plascencia-Hernández, A.L. Luna, C. Colbeau-Justin, P. Santiago, Garcia-Rocha, M., Valverde-Aguilar, G. M. A.Valenzuela. J. Saudi Chem. Soc, 23(8), 1016-1023,( 2019). DOI: 10.1016/j.jscs.2019.05.007
- R. Yang, X. Lu, X. Huang, Z. Chen, X. Zhang, M. Xu, Q. Song, L. Zhu. Appl Catal B, 170, 225-232,( 2015). DOI: 10.1016/j.apcatb.2015.01.046
- K. Chanda, S. Rej, M. H. Huang. Chem: Eur. J, 19(47), 16036-16043,( 2013). DOI: 10.1002/chem.201302065
- Q. Hua, T. Cao, X. K. Gu, J. Lu, Z. Jiang, X. Pan, L. Luo, W.X. Li, W. Huang. Angew Chem Int Ed Engl, 53(19), 4856-4861,( 2014). DOI: 10.1002/anie.201402374
- H. Zhang, Q. Zhu, Y. Zhang, Y. Wang, L. Zhao, B. Yu. Adv Funct Mater, 17(15), 2766-2771,( 2007). DOI: 10.1002/adfm.200601146
- Y. Xiong, B. Wiley, J. Chen, Z. Y. Li, Y. Yin, Y. Xia. Angew Chem Int Ed Engl, 44(48), 7913-7917,( 2005). DOI: 10.1002/anie.200502722
- D. F. Zhang, H. Zhang, L. Guo, K. Zheng, X. D. Han, Z. Zhang. J Mater Chem, 19(29), 5220-5225,( 2009). DOI: 10.1039/b816349a
- Z. Gao, B. Yao, T. Xu . Techno, 1:1-1,( 2019). DOI: 10.1080/09593330.2019.1670268
- W. Janusz, A. GAŁGAN, and M. RESZKA. PHYSICOCHEM PROBL MI, 40, 161-174,( 2006)
- N. I. C. K. Serpone, D. A. R. R. E. N. Lawless, R. Khairutdinov, E. Pelizzetti . J Phys Chem, 99(45), 16655-16661,(1995). DOI: 10.1021/j100045a027
- N. Daneshvar, D. Salari, and A. R. Khataee. J. Phys. Chem, 162(2-3), 317-322,(2004). DOI: 10.1016/S1010-6030(03)00378-2
- T. Sauer, G. C. Neto, H. J. Jose, R. F. P. M. Moreira. Photobiol. A, 149(1-3), 147-154,( 2002). DOI: 10.1016/S1010-6030(02)00015-1
- M. Kosmulsk. Adv Colloid Interface Sci, 152(1-2), 14-25,( 2009). DOI: 10.1016/j.cis.2009.08.003
- M. Kosmulsk, CRC pressm(145), 2009
- I. K. Konstantinou, T. A. Albanis. Appl Catal B, 49(1), 1-14,( 2004). DOI: 10.1016/j.apcatb.2003.11.010
- E. Bizani, K. Fytianos, I. Poulios, V. Tsiridis. J. Hazard. Mater, 10;136(1):85-94,( 2006). DOI: 10.1016/j.jhazmat.2005.11.017
- K. Tanaka, K. Padermpole, T. Hisanaga . Water Res, 34(1), 327-333,(2000). DOI: 10.1016/S0043-1354(99)00093-7
- C. Zhu, L. Wang, L. Kong, X. Yang, L. Wang, S. Zheng, H. Zong. Chemosphere, 41(3), 303-309,( 2000). DOI: 10.1016/s0045-6535(99)00487-7
- C. Hu, C. Y. Jimmy, Z. Hao, P. K. Wong. Appl Catal B, 46(1), 35-47,( 2003). DOI: 10.1016/S0926-3373(03)00139-5
- S. Maensiri, C. Masingboon, V. Promarak, and S. Seraphin. Opt Mater (Amst), 29(12), 1700-1705,( 2007). DOI: 10.1016/j.optmat.2006.09.011
- D. Bhatia, R. Kanwar, and J. Singh. dspace.lpu.in, 2018. DOI: 10.1016/j.jhazmat.2005.11.017
- F. Zhang, G. Dong , M. Wang, Y. Zeng, C. Wang. Appl. Surf. Sci,30,444,559-68,( 2018). DOI: 10.1016/j.apsusc.2018.03.087
- S. Karthikeyan, S. Kumar, LJ. Durndell, MA. Isaacs, CM. Parlett, B. Coulson, RE. Douthwaite, Z. Jiang, K. Wilson, AF. Lee. ChemCatChem, 21,10(16),3554-63,( 2018). DOI:10.1002/cctc.201800439
- R. Saadi, Z. Saadi, R. Fazaeli, and N. E. Fard. Korean J Chem Eng, 32(5), 787-799,(2015). DOI: 10.1007/s11814-015-0053-7
- J. F. Porter, G. McKay, and K. H. Choy. Chem Eng Sci, 54(24), 5863-5885,( 1999). DOI: 10.1016/S0009-2509(99)00178-5
- L. Nassaji-Jahromi, R. Fazaeli, R. Behjatmanesh-Ardakani, M.Taghdiri. J. Photochem. Photobiol. A, 392, 112425,(2020). DOI: 10.1016/j.jphotochem.2020.112425
- A. Seidel, D. Gelbin. Chem Eng Sci, 43(1), 79-88,( 1988). DOI: 10.1016/0009-2509(88)87128-8
- S. Agarwal, I. Tyagi, V. K. Gupta, F. Golbaz, A. N. Golikand, O. Moradi. J Mol Liq, 218, 494-498,( 2016). DOI: 10.1016/j.molliq.2016.02.040
- R. Bazargan-Lari, H. R. Zafarani, M. E. Bahrololoom, and A. Nemati. J Taiwan Inst Chem Eng, 45(4), 1642-1648,( 2014). DOI: 10.1016/j.jtice.2013.11.009
- B. H. Hameed. J Hazard Mater, 162(2-3), 939-944,( 2009). DOI: 10.1016/S0032-9592(02)00239-X
- A. Khaled, A. El Nemr, A. El-Sikaily, O. Abdelwahab. Desalination, 238(1-3), 210-232,(2009). DOI: 10.1016/j.desal.2008.02.014
- M. Özacar, İ. A. Şengil, H. Türkmenler. Chem Eng J, 143(1-3), 32-42,( 2008). DOI: 10.1016/j.cej.2007.12.005
- A. Almasian, M. Parvinzadeh Gashti, M. E. Olya, G. Chizari Fard. Desalin Water Treat, 57(44), 20837-20855,( 2016). DOI: 10.1080/19443994.2015.1112841
- Y. S. Ho, G. McKay. Water Res, 34(3), 735-742,(2000). DOI: 10.1016/S0043-1354(99)00232-8
- Y. S. Ho, G. McKay.Process Biochem, 38(7), 1047-1061,( 2003). DOI: 10.1016/S0032-9592(02)00239-X
- S. Schiewer, A. Balaria. Chem Eng J, 146(2), 211-219,( 2009). DOI: 10.1016/j.cej.2008.05.034
- S. Vafakhah, M. E. Bahrololoom, and M. Saeedikhani. J Water Resour Prot, 8(13), 1238-1250,( 2016). DOI: 10.4236/jwarp.2016.813095
- H. I. Chieng, L. B. Lim, N. Priyantha. Environ. Technol, 36(1), 86-97,( 2015). DOI: 10.1080/09593330.2014.938124
- X. Xiao, D. Liu, Y. Yan, Z. Wu, Z. Wu, G. Cravotto. J Taiwan Inst Chem Eng, 53, 160-167,( 2015). DOI: 10.1016/j.jtice.2015.02.031
- G. C. Fard, M. Mirjalili, F. Najafi. J Taiwan Inst Chem Eng, 70, 188-199,( 2017). DOI: 10.1016/j.jtice.2016.10.045

