- Gewald reaction,
- Thieno[2,
- 3-d]pyrimidines,
- 3-d]pyrimidin-2(1H)-ones,
- Antimicrobial activity
Copyright (c) 2017 Taslimahemad T. Khatri, Viresh H. Shah

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
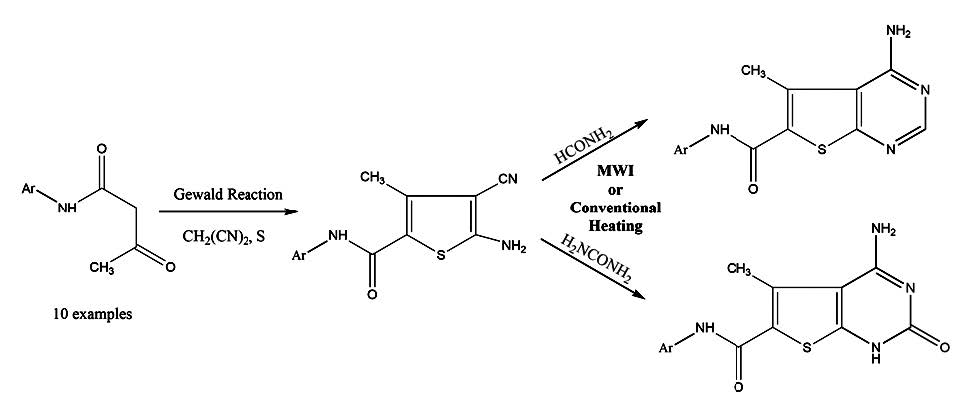
A series of novel 2-amino-3-cyanothiophenes (2a-2j) were synthesized using heterogeneous base (K2CO3) supported Gewald reaction. Cyclization of 2a-j with formamide and urea in conventional heating as well as microwave irradiation gave thieno[2,3-d]pyrimidines (3a-3j) and thieno[2,3-d]pyrimidin-2(1H)- ones(4a-4j) respectively. The reaction rates were faster and yields were higher in the microwave conditions. The structures of the compounds were confirmed with elemental analysis, mass spectral analysis, FTIR, 1H NMR and 13C NMR techniques. All the synthesized compounds were subjected to antimicrobial activity (MIC) in vitro by broth dilution method and exhibited a moderate antimicrobial activity.
References
- K. Gewald, Khim. Geterotsikl. Soedin. 1299 (1976).
- R.V. Sabnis, The gewald synthesis, Sulphur reports, 16, 1, (1994).
- L. Chengyuan, L. Dong, W. Xiuzhen, Z. Qingqing, Y. J. Qizheng, J. Sulfur. Chem. 34, 458 (2013).
- P. Zita, K. Alžbeta, V. Daniel, ARKIVOC, i, 209 (2010).
- R. M. Mahmoud, S.M.A. Fatma, T. A. Amira, M. A.Yasmeen, Synth. Commun. 45, 982 (2015).
- V. Alagarsamy, S. Meena, K. V. Ramseshu, V. R. Solomon, K. Thirumurugan, K. Dhanabal, M. Murugan, Eur. J. Med. Chem. 41, 1293 (2006).
- H.R. Roopa, J. Saravanan, S. Mohan, Rekha Parmesh, Asian J. Research Chem. 8(3), 213 (2015).
- A. A. El-Malah, A. E. Kassab, Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry, 14, 204 (2015).
- R. B. Toche, P. Nikam, Chemistry & Biology Interface, 5(4), 246 (2015).
- S. Bugge, I. U. Moen, K. K. Sylte, E. Sundby, B. H. Hoff, Eur. J. Med. Chem., 94, 175 (2015).
- L. D. Jennings, S. L. Kincaid, Y. D.Wang, G. Krishnamurthy, C. F. Beyer, J. P. Mginnis, M. Miranda, C. M. Discafani, S. K. Rabindran, Bioorg. Med. Chem. Lett. 15, 4731 (2015).
- M. D. Meyer, R. J. Altenbach, F. Z. Basha, W. A. Carroll, S. Condon, S. W. Elmore, J. F. Kerwin, K. B. Sippy, K. Tietje, M. D. Wendt, A. A. Hancock, M. E. Brune, S. A. Buckner, I. J. Drizin, Med. Chem. 43, 1586 (2000).
- D. H. Pandya, J. A. Sharma, H. B. Jalani, A. N. Pandya, V. Sundarsanam, S. Kachler, K. N. Klotz, K. Vasu, Bioorg. Med. Chem. Lett. 25, 1306 (2015).
- V. P. Litvinov, Russ.Chem.Bull., Int.Ed., 53, 487 (2004).
- A. I. Yehia, H. M. E. Ahmed, Adv. Heterocycl. Chem. 66, 235 (1996).
- A. S. Abd El-All, S. M. Sh. Atta, H. M. F. Roaiah, E. M. Awad, M. M. Abdalla, Arch. Pharm. 349, 202 (2016).
- M.M. Kandeel, H.M. Refaat, A.E. Kassab, I.G. Shahin, T.M. Abdelghany, Eur. J. Med. Chem. 90, 620 (2015).
- A.Ts. Mavrova, D. Wesselinova, J.A. Tsenov, L.A. Lubenov, Eur. J. Med. Chem. 86, 676 (2014).
- B. C. Shook, D. Chakravarty, J. K. Barbay, A. Wang, K.Leonard, V. Alford, M.T. Powell, S. Rassnick, R. H. Scannevin, K. Carroll, N. Wallace, J. Crooke, M. Ault, L. Lampron, L. Westover, K. Rhodes, P. F. Jackson, Bioorg. Med. Chem. Lett. 23, 2688 (2013).
- M.B. Dewal, A.S. Wani, C. Vidaillac, D. Oupicky´, M.J. Rybak, S.M. Firestine, Eur J Med Chem 51, 145 (2012).
- J. Clark, G. Hitiris, J. Chem. Soc. Perkin Trans. 1, 2005 (1984).
- K. M. Hassan, A. M. K. El-Dean, M. S. K. Youssef, F. M. Atta, M. S. Abbady, Phosphorus, Sulfur Silicon Relat. Elem. 47, 181 (1990).
- V. J. Ram, H. K. Pandey, A. J. Vlietinek, J. Heterocycl. Chem. 18, 1277 (1981).
- G. D. Madding, M. D. Thompson, J. Heterocycl. Chem. 24, 581 (1987).
- F. El-Telbany, R. O. Hutchins, J. Heterocycl. Chem. 22, 401 (1985).
- K. Kamlesh, K. Taslimahemad, P. Praful, J. Korean Chem. Soc. 57, 476 (2013).
- K. Taslimahemad, S. Viresh, J. Korean Chem. Soc., 58, 366 (2014).
- M. Kidwai, A. D. Mishra, Bull. Korean Chem. Soc. 24, 1038 (2003).
- National Committee for Clinical and Laboratory Standards, Method for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically Approved Standard, fourth ed. NCCLS, Villanova, Italy, Document M 100-S7. S100-S157 (1997).
- D. H. Isenberg, Essential Procedure for Clinical Microbiology, American Society for Microbiology, Washington, 1998.
- J. R. Zgoda, J. R. Porter, Pharm. Biol.39, 221 (2001).
- M. A. Abdelaziz, H. M. El-Sehrawi, R. M. Mohareb, Med Chem Res 24, 3932 (2015).
- M. H. Bhuiyan. K. M. Rahman, K. Hossain, A. Rahim, I. Hossain, M. Abu Naser, Acta Pharmaceutica. 56(4), 441 (2006).
- A. B. A. El-Gazzar, H. A. R. Hossein, H. N. Hafez, Acta Pharmaceutica. 57(4), 395 (2007).
- A. M. Farag, N. A. Kheder, K. M. Dawood, American Journal of Organic Chemistry, 5(2), 73 (2015).


