
- activated orange peels,
- isotherms,
- kinetics,
- magnetic alginate composite beads,
- methylene blue
Copyright (c) 2021 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
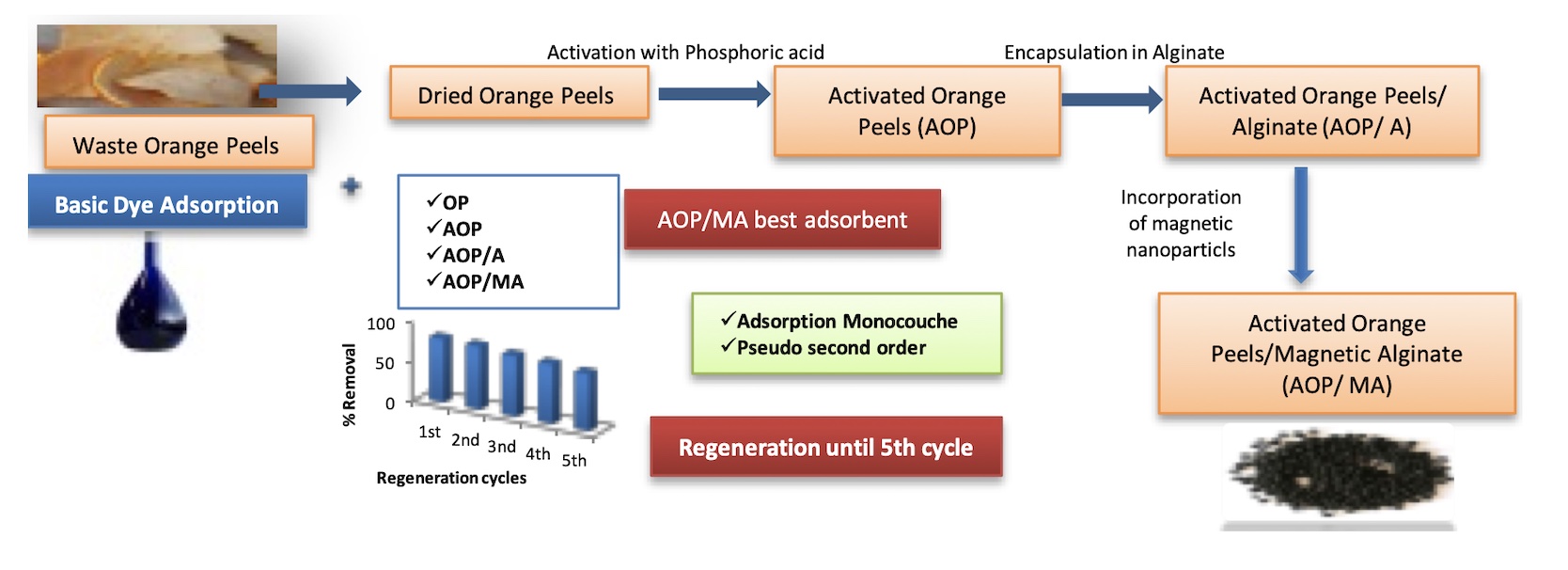
A comparative study for the adsorption of Methylene blue onto different prepared adsorbents was investigated. First, a comparative study was performed with different parameters between orange peels, orange peels activated with phosphoric acid, orange peels activated encapsulating in alginate and orange peels activated encapsulating in magnetic alginate. It can be concluded that the magnetic alginate composite beads were the best adsorbent. Then the isotherms, kinetics and regeneration studies for the removal of Methylene blue were studied onto magnetic alginate composite beads. The adsorption of Methylene blue on magnetic alginate composite beads was applied to isotherm models showed that the interaction of Methylene blue with magnetic alginate composite beads surface is localized monolayer adsorption. The kinetic process flow a pseudo-second-order kinetic. Finally, the removal efficiencies were maintained using HCl solution as desorbing agent after five cycles of adsorption-desorption.
References
- Aarfane, A.; Salhi, A.; El Krati, M.; Tahiri, S.; Monkade, M.; Lhadi, E.K.; and Bensitel, M. J. Mater. Environ. Sci. 5, 1927 (2014).
- Benhouria, A.; Islam, M.A.; Zaghouane-Boudiaf, H.; Boutahala, M.; and Hameed, B.H. Chem. Eng. J. 270, 621 (2015).
- Yao, Y.; Xu, F.; Chen, M.; Xu, Z.; and Zhu, Z. Bioresour. Technol.101, 3040 (2010).
- Peng, Y.; Wang, K.K.; Liu, T.; Xu, J.; and Xu, B.G. Appl. Catal. B. 203, 946 (2017).
- Pandey, A.; Singh, P.; and Iyengar, L. Int. Biodeter. Biodegr. 59, 73 (2017).
- Canizares, P.; Martínez, F.; Jiménez, C.; Lobato, J.; and Rodrigo, M.A. Environ. Sci. Technol. 40, 6418 (2006).
- Arslan, İ.; Balcioǧlu, A.D.; and Bahnemann, D.W. Dyes. Pigm., 47, 207 (2000).
- Al-Bastaki, N. Chem. Eng. Process. 43, 1561 (2004).
- Liu, Q.; Yang, B.; Zhang, L.; and Huang, R. Inter. J. Biol. Macromol. 72, 1129 (2015).
- Oladipo, A.A.; and Gazi, M. J. Water. Process. Eng. 2, 43 (2014).
- Peng, Q.; Liu, M.; Zheng, J.; and Zhou, C. Micropor. Mesopor. Mat. 201, 190 (2015).
- Kyzas, G.Z.; Lazaridis, N.K.; and Mitropoulos, A.Ch. Chem. Eng. J. 189, 148 (2012).
- Zeng, G.; Min, C.; Danlian, H.; Cui, L.; Piao, X.; Zhen, W.; Ningjie, L.; Chen, Z.; Xiaoxiao, H. and Yan, H. Waste. manage. 4, 424 (2015).
- Giannakoudakis, D.A.; Kyzas, G.Z.; Avranas, A.; and Lazaridis, N.K. J. Mol. Liq. 213, 381 (2016).
- Aichour, A.; Zaghouane-Boudiaf, H.; Iborra, C.V. and Polo, M.S. J. Mol. Liq. 256, 533 (2018).
- Hassani, A.; Soltani, R.D.C.; Karaca, S.; and Khataee, A. J. Ind. Eng. Chem. 21, 1197 (2015).
- Jost, V.; Kobsik, K.; Schmid, M.; and Noller, K. Carbohydr. Polym. 110, 309 (2014).
- Pankhurst, Q. A.; Connolly, J.; Jones, S. K.; Dobson, J. J. Phys. D Appl. Phys. 36, 167 (2003).
- Martin, C. R.; Mitchell, D. T. Anal. Chem. 70, 322 (1998).
- Rocher, V.; Siaugue, J.M.; Cabuil, V.; and Bee, A. Water. Res. 42, 1290 (2008).
- Fan, L.; Luo, C.; Sun, M.; Qiu, H.; and Li, X. Colloids. Surf. B. 103, 601 (2013).
- Travlou, N.A.; Kyzas, G.Z.; Lazaridis, N.K.; and Deliyanni, E.A. Langmuir. 29, 1657 (2013).
- Elwakeel, K.Z.; El-Bindary, A.A.; El-Sonbati, A.Z.; and Hawas, A.R. Can. J. Chem. 95, 807 (2017).
- Sun, L.; Chen, D.; Wan, S.; and Yu, Z. Bioresour. Technol. 198, 300 (2015).
- Zakaria, Z.A.; Suratman, M.; Mohammed, N.; and Ahmad, W.A. Desalination. 244, 109 (2009).
- Nasrullah, A.; Bhat, A.H.; Naeem A.; Isa, M.H.; and Danish, M. Int. J. Biol. Macromol. 107,1792 (2019).
- Ravi, R.; and Pandey, L.M.; Appl. Clay Sci. 169, 102 (2019).
- Alves, D.C.L.; Yáñez-Vilar, S.; Piñeiro-Redondo, Y.; and Rivas, J. Nanomaterials. 9, 356 (2019).
- Afroze, S.; Sen T.; Ang, H. M.; Nishioka, H. Desalin. Water. Treat. 57, 1 (2015).
- Shakoor, S.; Nasar, A. J. Taiwan. Inst. Chem. E. 66, 154 (2016).
- Hassan, A.F.; Abdel-Mohsen, A.M.; and Fouda, M.M.G. Carbohydr. Polym. 102, 192 (2014).
- Saadi, R.; Saadi, Z.; Fazaeli, R.; and Fard, N.E. Korean. J. Chem. Eng. 32, 787 (2015).
- Malik, P.K. J. Hazard. Mater. 113, 81 (2014).
- Hosseinia, M.; Mertensb, S.F.L.; Ghorbanic, M.; and Arshadi, M.R. Mater. Chem. Phys. 78, 800 (2003).
- Jovanovich, D.S. Colloid Polym. Sci., 235 1203 (1969).
- Inglezakisa, V.J.; and Zorpas, A.A. Desalin. Water. Treat. 39, 149 (2012).
- Auta, M. and Hameed, B.H. J. Ind. Eng. Chem. 19, 1153 (2013).
- Ho, Y.S.; and Mckay, G. J. Environ. Sci. Health. A34, 1179 (1999).
- Azizian, S.; Haerifar, M.; and Bashiri, H. Chem. Eng. J. 146, 36 (2009).
- Bhatti, H.N.; Zaman, Q.; Kausar, A.; Noreen, S.; and Iqbal, M. Eco.l Eng. 95, 216 (2016).


