SCREEN PRINTED ELECTRODE OF CARBON NANOTUBES MODIFIED WITH GOLD NANOPARTICLES FOR SIMULTANEOUS DETERMINATION OF ZINC, LEAD AND COPPER

- Gold Nanoparticles,
- Screen-Printed Electrode,
- Carbon Nanotubes
Copyright (c) 2020 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
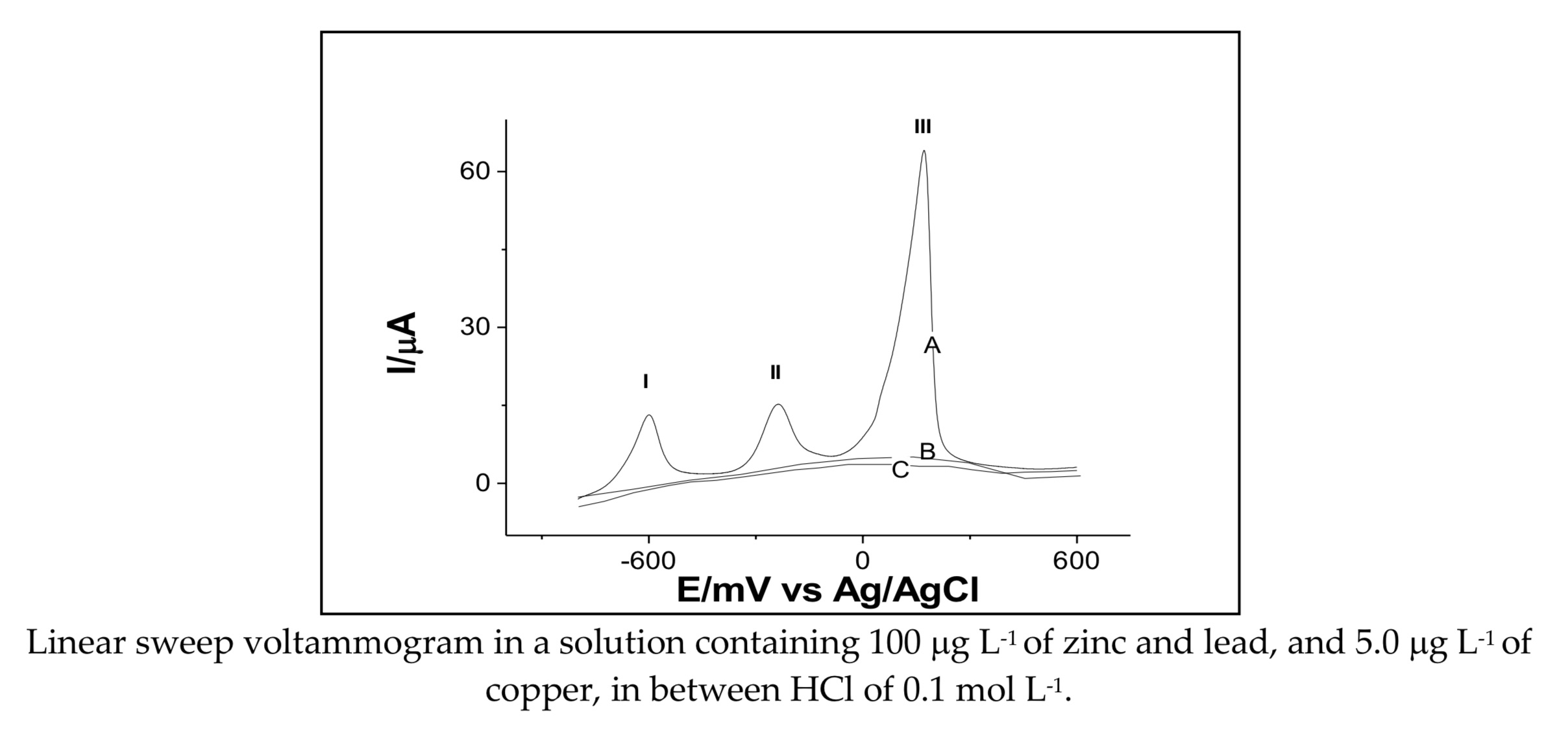
Differential pulse anodic stripping voltammetry, method used for simultaneous determination of zinc, copper and lead with screen printed electrode of carbon nanotubes modified with gold nanoparticles was investigated. The results indicate that a simultaneous identification and quantification of zinc, lead and copper is possible at -0.6 V, -0.26 V and 0.16 V respectively. The maximum current was linearly dependent on the concentration of Zn2+, Pb2+ and Cu2+, thus allowing the construction of analytical curves, being: -Ip (A) = 0.08 + 0.214 [Zn2+ (g L-1)] in a range of 9.9 to 120 mg L-1 with an R2 = 0.993; -Ip (A) = -1.06 + 0.240 [Pb2+ (mg L-1)] in a range of 9.9 to 120 mg L-1 with an R2 = 0.998; -Ip (A) = -10.93 + 15.16 [Cu2+ (mg L-1)] in a range of 0.99 to 12 mg L-1 with an R2 = 0.998. The limits of detection and quantification were estimated between 1.0 - 3.5 mg L-1 for Zn2+, 1.5 - 5.0 mg L-1 for Pb2+ and 0.1 - 0.33 mg L-1 for Cu2+. An integral, selective and reliable system for simultaneous determination of heavy metals was achieved, which may have applications in field monitoring for environmental use and public health.
References
- Behrman R.E. "Textoook of Pediatrics" 14 Ed., W.B. Saunders Company, Philadelphia, EE.UU, 1992, p. 1788-17791.
- Agency of Toxic Substances and Disease Registry. Case studies in environmental medicine. Lead toxicity. US Department of Health and Human Services, Public Health Service. Atlanta, GA: The Agency.
- Budd P, Montgomery J, Cox A, Krause P, Barreiro B, Thomas R.G. The distribution of lead within ancient and modern human teeth: implications for long-term and historical exposure monitorin. Sci Total Environ. 1998, Vol. 220, p. 121-136.
- Lanphear B.P.; Matte T.D., Rogers J.; Cickner R.P.; Dietz B.; Bornschein R.L., et al. The contribution of lead-contaminated house dust and residential soil to children’s blood lead levels. A pooled analysis of 12 epidemiologic studies. Environ Res. 1998, Vol. 79, p. 51-68.
- Guidelines for drinking wáter quality third edition, volumen 1. World health Organization.
- Malvankar P.L.; Shinde V.M. Ion-pair extraction and determination of copper (II) and zinc (II) in environmental and pharmaceutical simple. Analyst 1991, Vol.10, P. 1081-1084.
- Aitio A.; Aro A.; Jarvisalo J., Vainio H. Trace elements in health and disease,1 st ed ., RSC: Cambridge the royal society of chemistry 1991, Vol. 125, p. 141-155.
- Pellerano R.G.; Romero C.H.; Acevedo H.A. y Vázquez F.A. Un método de bajo costo para determinación de cobre a nivel de vestigios en matrices de interés ambiental por espectrofotometría en fase sólida. Quim. Nova 2007, Vol. 30, p. 2020-2024.
- Blanco Hernández A.L.; Alonso Gutiérrez D.; Jiménez de Blas O.; Santiago Guervós M.; Manzano B. Estudio de los niveles de plomo, cadmio, zinc y arsénico, en aguas de la provincia de salamanca. Rev Esp Salud Pública 1998, Vol 72, p. 53-65.
- Viñas P.; López-García I.; Lanzón M. and Hernández-Córdoba M. Direct Determination of Lead, Cadmium, Zinc, and Copper in Honey by Electrothermal Atomic Absorption Spectrometry using Hydrogen Peroxide as a Matrix Modifier. J. Agric. Food Chem. 1997, Vol. 45, p. 3952−3956.
- Mykytiuk A.P.; Russell D.S. and Sturgeon R.E. Simultaneous determination of iron, cadmium, zinc, copper, nickel, lead, and uranium in sea water by stable isotope dilution spark source mass spectrometry. Analytical chemistry 1998, Vol.52, p.1281-1283.
- Leermakers M.; Baeyens W.; Gieter M.D.; Smedts B.; Meert C.; De Bisschop H.C.; Morabito R.; Quevauviller P. Toxic arsenic compounds in environmental samples: speciation and validation. Trends Anal Chem. 2006, Vol. 25, p. 1–10.
- Hung D.Q.; Nekrassova O.; Compton R.G. Analytical methods for inorganic arsenic in water: a review. Talanta 2004, Vol. 64, p. 269–277.
- Gamboa J.C.M.; Peña R.C.; Paixão T.R.L.C.; Bertotti M. A renewable copper electrode as an amperometric flow detector for nitrate determination in mineral water and soft drink samples. Talanta 2009, Vol.80, p. 581-585.
- Hawkings R.C. and Thode H.G. Polarographic determination of copper, lead and cadmium in high-purity zinc alloys. Industrial and Engineering Chemistry 1944, Vol.16, p. 71-74.
- Barra C.M.; Dos Santos M.M.C. Speciation of inorganic arsenic in natural waters by square-wave cathodic stripping voltammetry. Electroanalysis 2001, Vol. 13, p.1098–1104.
- Greulach U.; Henze G. Analysis of arsenic(V) by cathodic stripping voltammetry. Anal Chim Acta .1995. Vol.306. p. 217–223.
- Wang J.; Lu J.; Hocevar S.B.; Farias PAM and Ogorevc B. Bismuth-coated carbon electrodes for anodic stripping voltammetry. Analytical Chemistry 2000, Vol. 72, p. 3218-3222.
- Economou A. Bismuth-film electrodes: recent developments and potentialities for electroanalysis. Trends in Analytical Chemistry 2005, Vol.24, p. 334-340.
- Charalambous A. and Economou A. A study on the utility of bismuth-film electrodes for the determination of In(III) in the presence of Pb(II) and Cd(II) by square wave anodic stripping voltammetry. Analytica Chimica Acta 2005, Vol. 547, p. 53-58.
- Guo Z.; Feng F.; Hou Y. and Jaffrezic-Renault N. Quantitative determination of zinc in milkvetch by anodic stripping voltammetry with bismuth film electrodes. Talanta 2005, Vol. 65, p. 1052-1055.
- Tarasova V.A. Voltammetric determination of thallium(I) at a mechanically renewed Bi-graphite electrode. Journal of Analytical Chemistry 2007, Vol. 62 (2), p. 157-160.

