
- Heterocyclic compounds,
- Nitrogen-based,
- Indole,
- Spiro-indole,
- Anticancer agents
Copyright (c) 2020 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
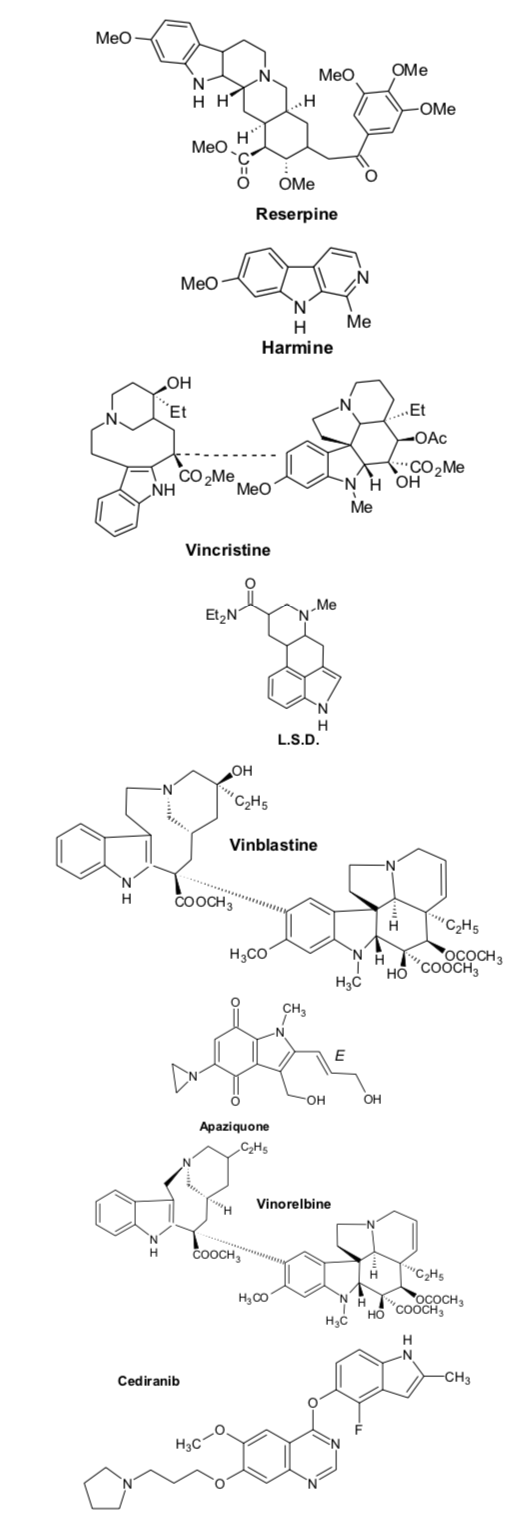
Abstract Heterocyclic moiety serve as perfect framework on which pharmacophores can be effectively attached to produce novel drugs. Among various heterocyclic compounds, nitrogen-based heterocycles have been extensively investigated as they constitute the core structures of numerous biologically relevant molecules and have been found to be active against different types of cancers. Due to the versatility of indole, it has been a highly “privileged motif” for the target-based design and development of anticancer agents. Moreover, it has been used as a synthon for the preparation of large number of bioactive heterocycles and paved a way to develop effective targets. This review article presents comprehensive overview of anticancer potentiality of diversely substituted indole derivatives including 1H-indole-2,3-dione and Spiro indole derivatives.
References
- V. V. Mulwad, B. P. Langi, A.C. Chaskar, Acta Pol Pharm 68, 39, ( 2011).
- A. Ansari, A. Ali, M Asif , New Journal of Chemistry 41, 16, (2017).
- M. Asif , Current medicinal chemistry 19, 2984, (2012).
- S. Biswal, U. Sahoo, S. Sethy, H.K. Kumar, M. Banerjee, Asian J Pharm Clin Res 5, 1, (2012).
- M. A. Chiacchio, D. Iannazzo, R. Romeo, S. V. Giofrè, L. Legnani, Current medicinal chemistry 26, 7166, (2019).
- J. H. Skerritt, and G. A. Johnston, Eur J Pharmacol 89, 193, (1983)
- R. Elliott, Mayo Clin Proc 76, 511, (2001).
- P. I. Joyce, D. Rizzi, G. Calo, D. J. Rowbotham and D. G. Lambert, Anesth Analg 95, 1339, (2002).
- E. Mutschler and H. Derendorf, Med. Pharm Scientific Publishers, Stuttgart, 16, (1995).
- Guyton and Hall, “Text book of Medical Physiology” Elsevier-Saunders, 7th Ed (2005).
- L. P. Garrod, Br. Med. J., 1 (5172), 527, (1960).
- C. H. Dash, Penicillin allergy and the cephalosporins. J Antimicrob Chemother, 1, 107, (1975).
- E. Goldstein, Am J Med 82, 3, (1987).
- W. Kingston, J History Med Allied Sci 59, 441 (2004).
- S. Senapati, A. K. Mahanta, S. Kumar, P. Maiti, Signal transduction and targeted therapy, 3, 1, (2018)
- V. Mistiaen, Time and the great healer. The history behind the discovery of streptomycin (2002).
- P. Martins, J. Jesus, S. Santos, L. R. Raposo, C Roma-Rodrigues, P. V. Baptista, A. R. Molecules 20,16852, (2015).
- S. Kakkar, S. Kumar, B. Narasimhan, S. M. Lim, K. Ramasamy, V. Mani, S. A. Shah, Chemistry Central Journal 12, 96, (2018).
- A. Kumari, R. K. Singh, Bioorganic chemistry 103021, (2019).
- S. J. Singh, R. Singla, V. Jaitak, Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents) 16, 160, (2016)
- M. Z. Zhang, Q. Chen, G. F. Yang, European journal of medicinal chemistry 89, 421, (2015).
- (a) T. V. Sravanthi, S. L. Manju, European Journal of Pharmaceutical Sciences 91, 1, (2016), (b) S. Dadashpour, S. Emami, European journal of medicinal chemistry, 150, 9, (2018).
- M. E. Welsch, S. A. Snyder, B.R. Stockwell, Curr Opin Chem Biol 14, 347, (2010).
- A. de Sa, R. Fernando, E. J. Barreiro, C.A. Manssour Fraga, Mini Rev Med Chem, 9, 782, (2009).
- B.E. Evans, K.E. Rittle, M.G. Bock, R.M. DiPardo, R.M. Freidinger, W.L. Whitter, G.F. Lundell, D.F. Veber, P.S. Anderson, R.S. Chang, V.J. Lotti, D.J. Cerino, T.B. Chen, P.J. Kling, K.A. Kunkel, J.P. Springer, J. Hirshfield, J. Med. Chem., 31, 2235, (1988).
- S. Verma, Y. S Prabhakar, Current medicinal chemistry. 22, 1603, (2015).
- D. Sunil, P. R. Kamath, Current topics in medicinal chemistry. 17, 959, (2017).
- D. L. Nelson, M. M. Cox, Principles of Biochemistry (4th Ed.), New York: W.H. Freeman, ISBN 0-7167-4339-6 (2005).
- C. Won, X. Shen, K. Mashiguchi, Z. Zheng, X. Dai, Y. Cheng, H. Kasahara, Y. Kamiya, J. Chory, Y. Zhao, Proc Natl Acad Sci U S A 108, 18518, (2011).
- M. Z. Zhang, N. Mulholland, D. Beattie, D. Irwin, Y.C. Gu, Q. Chen, G.F. Yang,
- J. Clough, Eur J Med Chem, 63, 22, (2013).
- H. A. Hamid, A. N. Ramli, M. M. Yusoff, Frontiers in pharmacology 8, 96, (2017).
- D. P. Mishra, M. A. Khan, D. K. Yadav, A. K. Rawat, R. K. Singh, T. Ahamad, M. K. Hussain, M. Saquib, M. F. Khan, Chemistry Select 3, 8468, (2018).
- W. Gul, M. T. Hamann, Life sciences 78, 442, (2005).
- J. W. Blunt, B. R. Copp, R. A. Keyzers, M. H. Munro, M. R. Prinsep, Natural Product Reports 34, 235, (2017).
- F.-E. Chen, J. Huang, Chem Rev 105, 4671, (2005).
- E. Stempel, T. Gaich, Accounts of chemical research 49, 2390, (2016).
- J. Zhou, J. H. Feng, L. Fang, Bioorganic & medicinal chemistry letters 27, 893 (2017).
- M. J. Ferreira, A. Paterna, Phytochemistry Reviews 18, 971, (2019).
- H. Ishikawa, D.A. Colby, D.L. Boger, J Am Chem Soc 130, 420, (2008).
- M. A. Jordan and L. Wilson, Nat. Rev. Cancer 4, 253, (2004).
- S. Suzen, Current Organic Chemistry 21, 2068, (2017).
- L. R. Chen, Y. C. Wang, Y. W. Lin, S. Y. Chou, S. F. Chen, L.T. Liu, Y. T. Wu, C. J. Kuo, T. S. Chen, and S. H. Juang, Bioorg Med Chem Lett 15, 3058, (2005).
- T. R. Bal, B. Anand, P. Yogeeswari and D. Sriram, Bioorg Med Chem Lett, 15, 4451, (2005).
- O. Guzel, N. Karali and A. Salman, Bioorg Med Chem, 16, 8976, (2008).
- S. Smitha, S. N. Pandeya, J. P. Stables, and S. Ganapathy, Sci. Pharm 76, 621, (2008).
- D. Banerjee, P. Yogeeswari, P. Bhat, A. Thomas, M. Srividya, and D. Sriram, Eur. J. Med. Chem 46, 106, (2011).
- T. Patel, R. Gaikwad, K. Jain, R. Ganesh, Y. Bobde, B. Ghosh, K. Das, S. Gayen, ChemistrySelect 4, 4478, (2019).
- K. Kaur, V. Jaitak, Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents), 19, 962, (2019).
- V. K. Rao, B. S. Chhikara, A. N. Shirazi, R. Tiwari, K. Parang, A. Kumar, Bioorganic & medicinal chemistry letters 21, 3511, (2011).
- N. V. Lakshmi, P. Thirumurugan, K. M. Noorulla, P. T. Perumal, Bioorganic & medicinal chemistry letters 20, 5054 (2010).
- A. S. Gurkan-Alp, M. Mumcuoglu, C. A. Andac, E. Dayanc, R Cetin-Atalay, E. Buyukbingol, European journal of medicinal chemistry. 58, 346, (2012).
- S. Safe, S. Papineni, S. Chintharlapalli, Cancer letters 269, 326, (2008).
- J. R. Weng, C. H. Tsai, S. K. Kulp, C. S. Chen, Cancer letters 262, 153, (2008).
- V. P. Garikapaty, B. T. Ashok, Y. G. Chen, A. Mittelman, M. Iatropoulos, R. K. Tiwari, Oncology reports 13, 89, (2005).
- S. A. Patil, R. Patil, D. D. Miller, Future medicinal chemistry 4, 2085, (2012).
- A. Andreani, S. Burnelli, M. Granaiola, A. Leoni, A. Locatelli, R. Morigi, M. Rambaldi, L.Varoli, L. Landi, C. Prata, M. V. Berridge, Journal of medicinal chemistry 51, 4563, (2008).
- J. Amato, N. Iaccarino, B. Pagano, R. Morigi, A. Locatelli, A. Leoni, M. Rambaldi, P. Zizza, A. Biroccio, E. Novellino, A. Randazzo, Frontiers in chemistry 2, 54, (2014).
- V. Sharma, R. Kalia, T. Raj, V. K. Gupta, N. Suri, A. K. Saxena, D. Sharma, S. S. Bhella, G. Singh, M. P. Ishar, Acta pharmaceutica Sinica B 2, 32, (2012).
- S. H. Zhuang, Y.C. Lin, L.C Chou, M. H. Hsu, H. Y. Lin, C. H. Huang, J. C. Lien, S. C. Kuo, L. J. Huang, European journal of medicinal chemistry 66, 466, (2013).
- S. A. Patil, J. K. Addo, H. Deokar, S. Sun, J. Wang, W. Li, D. P. Suttle, W. Wang, R. Zhang, J. K. Buolamwini, Drug designing: open access 6, (2017).
- I. Ali, S. D. Mukhtar, M. F. Hsieh, Z. A. Alothman, A. Alwarthan, RSC advances 8, 37905, (2018).
- M. M. Kamel, M. K. Abdel-hameid, H. B. El-Nassan, E. A. El-Khouly, Medicinal Chemistry 15, 873, (2019).
- S. Cascioferro, G. Li Petri, B. Parrino, B. El Hassouni, D. Carbone, V. Arizza, U. Perricone, A. Padova, N. Funel, G. J. Peters, G. Cirrincione, Molecules 25, 329, (2020).
- H. Kaur, J. Singh, B. Narasimhan, BMC chemistry 13, 65, (2019).
- K. L. Vine, L. Matesic, J. M. Locke, M. Ranson, D. Skropeta, Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents) 9, 397, (2009).
- N. Ristovska, F. Anastasova, M. Stefova, Molbank 2013(2), M798, (2013).
- P. Pakravan, S. Kashanian, M. M. Khodaei, F. J. Harding, Pharmacological Reports. 65, 313, (2013).
- R. Jarapula, K. Gangarapu, S. Manda, S. Rekulapally, International journal of medicinal chemistry 2016, (2016).
- P. K. Shukla, M. P. Singh, R. Patel, Journal of Applied Pharmaceutical Sciences and Research 16 (2018).
- O. L. Erdman, Journal für Praktische Chemie 19, 321, (1840).
- A. Laurent, Ann Chim Phy 3, 393, (1840).
- A. A. Jarrahpour and D. Khalili, Molbank, M437 (2005).
- K.A.J.A.L Bayati, Tikrit J. Pure Sci, 2, 17, (2012).
- S. N. Pandeya, P. Yogeeswari, D. Sriram and G. Nath, Bull Chim Farm 137, 321, (1998).
- M. Sarangapani and V. M. Reddy, Indian J Heterocycl Chem 3, 257, (1994).
- R. S. Varma and W. L. Nobles J Pharm Sci 64, 881, (1975).
- S. K. Sridhar, S. N. Pandeya, J. P. Stables and A. Ramesh Eur J Pharm Sci 16, 129, (2002).
- M. Varma, S. N. Pandeya, K. N. Singh and J. P. Stables Acta Pharm 54, 49, (2004).
- S. N. Pandeya, D. Sriram, G. Nath and E. De Clercq Acta Helv 74, 11, (1999).
- S. N. Pandeya, D. Sriram, G. Nath, and E. De Clercq, Eur J Med Chem 35, 249 (2000).
- S. N. Pandeya, D. Sriram, G. Nath, and E. De Clercq, Arzneim-Forsch Drug Res 50, 55, (2000).
- S. P. Singh, S. K. Shukla, and L. P. Awasthi, Curr Sci 52, 766 (1983).
- S. S. Karki, U. K. Mazumder, M. Gupta, S. Bhattacharya, S. Rathinasamy, and S. Thangavel, Chem Pharm Bull 52, 178, (2004).
- S. S. Karki, T. Shrikanth, Y. D. Satyanarayana, J. Balzarini, and E. De Clercq, Bioorg Med Chem 15, 6632, (2007).
- K. C. Joshi, V. N. Pathak, and S. K. Jain, Pharmazie 35(11), 677, (1980).
- K. C. Joshi, V. N. Pathak, and R. K. Chaturvedi, Pharmazie 41, 634, (1986).
- R. Jain and M. A. Bansal, Pharmazie 50, 224, (1995).
- K. L. Vine, J. M. Locke, M. Ranson, S. G. Pyne, J. B. Bremner Bioorganic & medicinal chemistry 15, 931, (2007).
- Y. O. Teng, H. Y. Zhao, J. Wang, H. Liu, M. L. Gao, Y. Zhou, K. L. Han, Z. C. Fan Y. M. Zhang, H. Sun, P. Yu, European journal of medicinal chemistry 112, 145, (2016).
- S. B. KumarM, M. Ravinder, G. Kishore, V. J. Rao, P. Yogeeswari, D. Sriram, Medicinal Chemistry Research 23, 1934, (2014).
- R. Gudipati, R. N. Anreddy, S. Manda Saudi Pharmaceutical Journal 19, 153, (2011).
- A. Kamal, Y. V. Srikanth, M. N. Khan, T. B. Shaik, M. Ashraf, Bioorganic & medicinal chemistry letters 20, 5229, (2010).
- N. M. Evdokimov, I. V. Magedov, D. McBrayer, A. Kornienko, Bioorganic & medicinal chemistry letters 26, 1558, (2016).
- W. M. Eldehna, M. F. Abo-Ashour, A. Nocentini, P. Gratteri, I. H. Eissa, M. Fares, O. E. Ismael, H. A. Ghabbour, M. M. Elaasser, H. A. Abdel-Aziz, C. T. Supuran, European journal of medicinal chemistry 139, 250, (2017).
- A. Nagarsenkar, L. Guntuku, S. D. Guggilapu, S. Gannoju, V. G. Naidu, N. B. Bathini European journal of medicinal chemistry. 124, 782, (2016).
- H. S. Ibrahim, S. M. Abou-Seri, H. A. Abdel-Aziz, European journal of medicinal chemistry 122, 366, (2016).
- L. Zhang, F. Chen, J. Wang, Y. Chen, Z. Zhang, Y. Lin, X. Zhu, RSC Advances. 5, 97816, (2015).
- T. Nasr, S. Bondock, M. Youns, European journal of medicinal chemistry 76, 539, (2014).
- R. Kakkar, MedChemComm 10, 351, (2019).
- K. C. Joshi, R. Jain, and P. Chand, Heterocycles, 23, 957, (1985).
- A. Dandia, R. Singh, H. Sachdeva, R. Gupta, S. Paul, Journal of the Chinese
- Chemical Society, 50, 273, (2003).
- A. Nandakumar, P. Thirumurugan, P. T. Perumal, P. Vembu, M. N.
- Ponnuswamy, P. Ramesh, Bioorganic & medicinal chemistry letters.
- , 4252, (2010).
- T. H. Kang, K. Matsumoto, M. Tohda, Y. Murakami, H. Takayama, M.
- Kitajima, N. Aimi, H. Watanabe, European journal of pharmacology 444, 39,
- (2002).
- A. A. Patchett, R. P. Nargund, J. R. Tata, M. H. Chen, K. J. Barakat, D. B.
- Johnston, K. Cheng, W. W. Chan, B. Butler, G. Hickey, Proceedings of the
- National Academy of Sciences 92, 7001, (1995).
- R. Misra, R. C. Pandey, J. V. Silverton, Journal of the American Chemical
- Society 104, 4478, (1982).
- P. Saraswat, G. Jeyabalan, M. Z. Hassan, M. U. Rahman, N. K. Nyola,
- Synthetic Communications 46, 1643, (2016).
- K. C. Joshi, A. Dandia, S. Bhagat, Journal of Fluorine Chemistry 48, 169,
- (1990).
- M.S. Islam, H. M. Ghawa, F. F. El-Senduny, A. M. Al-Majid, Y. A. Elshaier,
- F. A Badria, A. Barakat, Bioorganic chemistry 82, 423, (2019).
- A. Barakat, M. S. Islam, H. M. Ghawas, A. M. Al-Majid, F. F. El-Senduny,
- F. A. Badria, Y. A. Elshaier, H. A. Ghabbour, RSC advances 8, 14335,
- (2018).
- D. Kaminskyy, D. Khyluk, O. Vasylenko, L. Zaprutko, R. Lesyk, Scientia
- pharmaceutica. 79, 763, (2011).
- C. Han, T. Zhang, A. Zhang, D. Wang, W. Shi, J. Tao, Synthesis.
- , 1389, (2014).
- D. Konyar, C. A. Andac, E. Buyukbingol, Letters in Drug Design &
- Discovery 15, 37, (2018).
- S. R. Yong, A. T. Ung, S. G. Pyne, B. W. Skelton, A. H. White, Tetrahedron.
- , 5579, (2007).
- A. Barakat, M. S. Islam, H. M. Ghawas, A. M. Al-Majid, F. F. El-Senduny, F.
- A. Badria, Y. A. Elshaier, H. A. Ghabbour, RSC advances 8, 14335,
- (2018).
- M. S. Islam, H. M. Ghawas, F. F. El-Senduny, A. M. Al-Majid, Y. A. Elshaier
- F. A. Badria, A. Barakat, Bioorganic chemistry 82, 423, (2019).
- L. Wu, Y. Liu, Y. Li, Molecules 23, 2330, (2018).
- G. Lotfy, M. M. Said, H. El Sayed, H. El Sayed, A. Al-Dhfyan, Y. M. Aziz,
- A. Barakat, Bioorganic & medicinal chemistry 25, 1514, (2017).
- N. Arumugam, A. I. Almansour, R. S. Kumar, D. M. Al-Thamili, G. Periyasami, V. S. Periasamy, J. Athinarayanan, A. A. Alshatwi, S. M. Mahalingam, J. C. Menéndez, Chemistry Central Journal 12, 1, (2018).
- W. M. Eldehna, D. H. EL-Naggar, A. R. Hamed, H. S. Ibrahim, H. A. Ghabbour, H. A. Abdel-Aziz, Journal of enzyme inhibition and medicinal chemistry, 33, 309, (2018).
- A. A. Patravale, A. H. Gore, G. B. Kolekar, M. B. Deshmukh, P. B. Choudhari, M. S. Bhatia, S. Prabhu, M. D. Jamdhade, M. S. Patole, P. V. Anbhule, Journal of the Taiwan Institute of Chemical Engineers 68, 105, (2016).

