VIBRATIONAL SPECTRUM CHARACTERIZATION OF OUTER SURFACE OF HELICOBACTER PYLORI BIOFILMS BY FUNCTIONALLY-ENHANCED DERIVATIVE SPECTROSCOPY (FEDS)

- Helicobacter pylori,
- infrared spectrum,
- biofilm,
- FEDS transform,
- surface sensing
Copyright (c) 2020 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
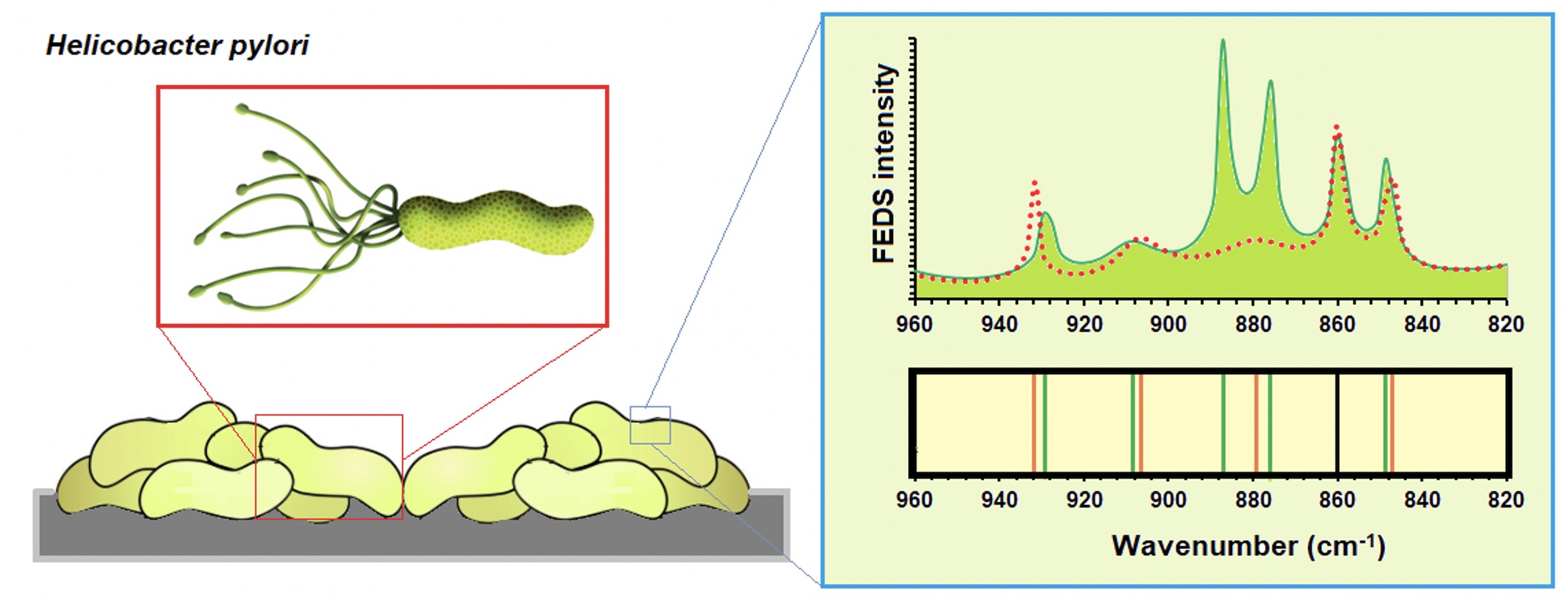
Mid-infrared spectroscopy in conjunction with Functionally-Enhanced Derivative Spectroscopy (IR+FEDS) is a powerful analytical tool for the improving of analysis of microorganism IR spectra. The objective of this work is to characterize the outer surface of two H. pylori strains by IR+FEDS. This work is a key stage for the study of cell-cell and cell-surface interactions between microorganisms, as well as, for polymicrobial biofilm characterizations where H. pylori species are involved. For that, artificial bacterial biofilms, or bacterial biolayers, were deposited on ultrafiltration cellulose membranes which were previously modified by covalent insertion of a spectral marker and used as sensing surface for analysis of bacterium biolayers. Biolayers were analyzed using an infrared spectrophotometer with ATR and data were analyzed by classic procedures and by deconvolution based on FEDS transform. It is concluded that, for correct application of technique is required a minimum amount of noise in the working spectra which can be achieved by simple smoothing algorithms; in addition, reproducibility must be warranted by the implementation of standardized protocols and the use of an appropriate number of samples. It is concluded that in addition to typical signals associated with IR spectrum of microorganisms, by FEDS, a better and more detailed description of outer membrane of H. pylori biofilms can be performed. In particular, the detecting and monitoring of cysteine-rich proteins can be satisfactorily performed by IR+FEDS.
References
- S. Phandis, M. Parlow, M. Levy, D. Ilver, C. Caulkins, J. Connors, B. Dunn, Infect. Immun., 64, 905 (1996).
- M. Chmiela, E. Czkwianianc, T. Wadstrom, W. Rudnicka, Gut 40, (1997).
- A.M. Alkout, C.C. Blackwell, D.M. Weir, I.R. Poxton, R.A. Elton, W. Luman, K. Palmer, Gastroenterol., 112, 1179 (1997).
- G. Sachs, D. Scott, D. Weeks, K. Melchers, Trends Microbiol., 9, 532 (2001).
- S.L. Palencia, G. Lagos, A. García, J. Sci. Technol. Appl., 1, 53 (2016).
- V. Marqués, B. Cunha, A. Couto, P. Sampaio, LP. Fonseca, S. Aleixo, CRC. Calado, Spectrochim. Acta A: Mol. Biomol. Spectr., 210, 193 (2018).
- H. Zhao, Y. Wu, Z. Xu, R. Ma, Y. Ding, X. Bai, Q. Rong, Y. Zhanng, B. Li, X. Ji, Front. Microbiol., 10, 1 (2019).
- G. Bode, F. Mauch, H. Ditschuneit, P. Malfertheiner, J. General Microbiol., 139, 3029 (1993).
- B.E. Dunn, N.B. Vakil, B.G. Schneider, M.M. Miller, J.B. Zitzer, T. Peutz. Infection and Immunology., 65, 1181 (1997).
- M.R. Amievalow, E.M. El-Omar, Gastroenterol., 134, 306 (2008).
- H. Shimomura, K. Hosoda, S. Hayashi, K. Yokota, Y. Hirai, J. Bacteriol., 194, 2658 (2012).
- A. Moran. FEMS Immunol. Medical Microbiol., 10, 271 (1995).
- N. Sabarth, S. Lamer, U.Zimny-Amdt, P.R. Jungblut, T.F. Meyer, D. Bumann. J. Biol. Chem., 277, 27896 (2002).
- H.H. Tuson, D.B. weibel. Soft Matter. 9, 4368 (2013).
- S. Kumar, A.K. Rai, V.B. Singh, S.B. Rai. Spectrochim. Act Part A: Mol. Biomol. Spectr. 61, 2741 (2005).
- M. Fischer, G.J. Triggs, T.F. Krauss. Appl Environ. Microbiol. 82, 1362 (2016).
- A. Elbourne, J. Chapman, A. Gelmi, D. Cozzolino, R.J. Crawford, V.K. Truong. J. Colloid Interf. Sci. 546, 192-210 (2019).
- F. Abbasian, E. Ghafar, S. Magierowsky. Bioengineering (Basel). 5, 1 (2018).
- A. Alvarez-Ordoñez, D. Mouwen, M. López, M. Prieto, J. Microbiol. Met. 84, 369 (2011).
- J. Ojeda, M. Dittrich in: Microbial systems biology: Methods and Protocols, Methods in Molecular Biology, Navid A. Springer Science, 2012, pp. 187-211.
- J. Prakash, S. Kar, C. Lin, C.Y. Chen, C.F. Chang, J.S. Jean, T.R. Kulp. Spectrochim. Act Part A: Mol. Biomol. Spectr. 116, 478-484 (2013).
- W. Friesen, K. Michaelian, Appl. Spectr. 45, 50-56 (1991).
- T. Vazhnova, D. Lukyanov. Anal. Chem. 85, 11291-11296 (2013).
- M. Palencia, J. Adv. Res.14, 53 (2018).
- M. Palencia, T. Lerma, N. Afanasjeva. Eur. Polym. J.115, 212 (2019).
- A. Otalora, M. Palencia, J. Sci. Technol. Appl. 6, 96 (2019).
- L.R. Anaya, K.H. Libreros, V.J. Palencia, V.J. Atencio, M. Palencia. J. Sci. Technol. Appl. 6, 96 (2019).
- Y. Guan, C.J. Wurrey, G.J. Thomas. Biophys. J., 66, 225 (1994).
- Y. Guan, G.J. Thomas. Biopolym. 39, 813 (1996).
- W. Jiang, A. Saxena, B. Song, B.B. Ward, T.J. Beveridge, S. Myneni. Langmuir. 20, 11433 (2004).
- E. Pretsch, C. Bühlmann, C. Affolter, C. Herrera, R. Martínez, Determinación estructural de compuestos orgánicos, Barcelona, Ed. Masson., 2005, 481 p.
- F.O. Libnau, O.M. Kvalheim, A.A. Christy, J. Toft. Vibr. Spectr. 7, 243 (1994).
- E. Wiercigroch, E. Szafraniec, K. Czamara, M.Z. Pacia, K. Majzner, K. Kochan, A. Kaczor, M. Baranska, K. Malek. Spectrochim Acta Part A: Molec Biomol Spectr. 185, 317 (2017).
- A. Kovacs, B. Nyerges, V. Izvekov. J Phys Chem., 112, 5728 (2008).
- A. Moran. FEMS Immunol. Medical Microbiol., 10, 271 (1995).
- A. Moran, In: Helicobacter pylori: Physiology and Genetics. Mobley HLT, Mendz GL, Hazell SL (Eds). ASM Press. Washington DC, USA, 2001.
- S. Parker. Chem. Phys. 424, 75-79 (2013).
- C. Dumrese, L. Slomianla, U. Ziegler, S.S. Choi, A. Kalia, A. Fulujira, W. Lu, D. Berg, M. Benghezal, B. Marshall, P. Mittl. FEBS. Lett. 583, 1637 (2009).
- R.A. Alm, J. Bina, B. Andrews, P. Doig, R. Hanckck, T. Trust, Infect Immun., 68, 4155 (2000).
- P. Cao, M.S. McClain, M.H. Forsyth, T. Cover. Infect. Immun., 66, 2984 (1998).
- P.R. Mittl, L. Luthy, P. Hunziker, M. Grutter. The J. Biol. Chem., 275, 17693 (2000).
- G. Zanottu, L. Cendron. Worl J. Gastroenterol., 20, 1402 (2014).
- E. Pretsch, C. Bühlmann, C. Affolter, C. Herrera, R. Martínez, “Determinación estructural de compuestos orgánicos”, Barcelona, Ed. Masson., 2005.
- C.V. Stephenson, W.C. Coburn, W.S. Wilcox. Spectrochim. Acta., 17, 933-946 (1961).
- A. Heidari, Austin J. Analyt. Pharm. Chem., 3, 1058 (2016)
- V. Andrushchenko, L. Benda, O. Pav, M. Dracinsky, P. Bour. The J. Phys. Chem B. 119, 10682 (2015).

