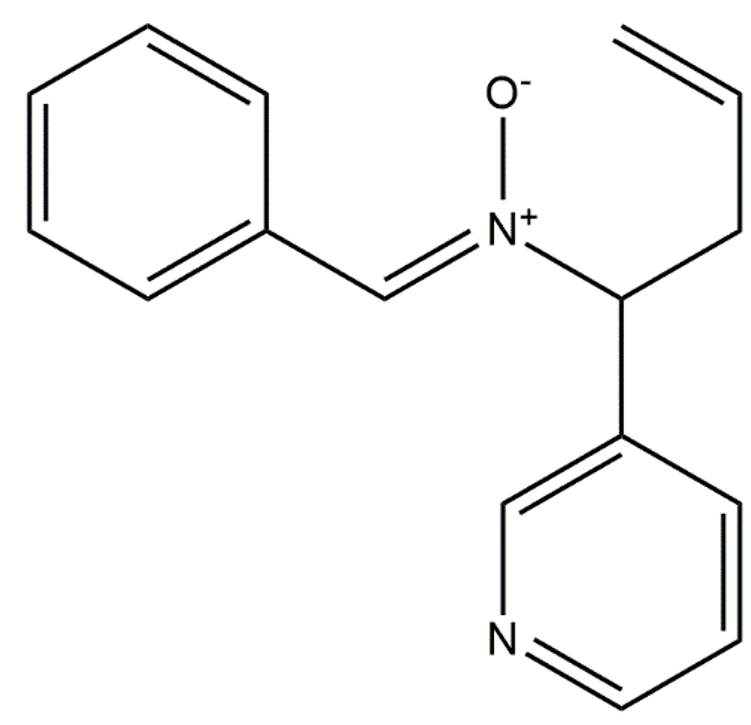
CRYSTAL STRUCTURE, HIRSHFELD SURFACE ANALYSIS AND ENERGY FRAMEWORK STUDY OF THE NITRONE N-BENZYLIDENE-N-BUTYLAMINO-4-Β-PYRIDYL-N-OXIDE

- nitrone,
- 1,3-dipolar cycloaddition,
- X-ray crystal structure,
- hydrogen bonds,
- Hirshfeld
Copyright (c) 2020 SChQ

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
The title compound, C16H16N2O, a potential antiparasitic agent, crystallizes in the orthorhombic Pca21 space group with unit cell parameters a= 9.912(1) Å, b= 9.035(1) Å, c= 15.681(2) Å. The crystalline structure is stabilized by weak C---H···O and C--H···Cg(π) interactions among neighboring molecules producing an efficient packing with 66.0% of occupied space. The C--H···O hydrogen bond keeps the molecules linked into supramolecular chains propagating along the a axis direction with a graph-set notation C(4), which are reinforced by C--H···Cg(π) interactions. Hirshfeld surface analysis of the intermolecular contacts reveal that the most important contributions for the crystal packing are from H··H (55.2%) and H··C/C··H (27.1%) interactions. Energy framework calculations suggest that the contacts formed between molecules are slightly dispersive in nature.
References
- V. V. Kouznetsov, L. Vargas-Méndez, F. I. Zubkov, Min. Rev. Org. Chem. 13, 488 (2018).
- L. Luna, L. Vargas-Méndez, V. V. Kouznetsov, Org. Med. Chem. Int. J. 7, 555708, (2018).
- M. Acelas, V. V. Kouznetsov, A. R. Romero-Bohórquez, Mol. Diversity, 23, 183, (2019).
- M. Breugst, R. Huisgen, H. U. Reissig, Eur. J. Org. Chem. 20, 2477, (2018).
- R. A. Miranda-Quintana, P. W. Ayers, Theor. Chem. Acc. 135, 172, (2016).
- R. A. Miranda-Quintana, M. Martínez-González, D. Hernández-Castillo, L. A. Montero-Cabrera, P. W. Ayers, C. Morell, J. Mol. Model. 23, 236, (2017).
- A. Padwa, S. Bur, Adv. Heterocycl. Chem. 119, 241, (2016).
- N. A. Bokach, M. L. Kuznetsov, V. Y. Kukushkin, Coord. Chem. Rev. 255, 2946, (2011).
- K. V. Gothelf, K. A. Jørgensen, Chem. Rev. 98, 863-910 (1998).
- A. Varlamov, V. V. Kouznetsov, F. Zubkov, A. Chernyshev, O. Shurupova, L. Y. Vargas, A. Palma, J. Rivero, A. J. Rosas-Romero, Synthesis, 29, 771, (2002).
- V. V. Kouznetsov, J. Rivero, C. Ochoa, E. E. Stashenko, J. R. Martínez, C. Ochoa, D. Montero, J. J. Nogal, C. Fernández, S. Muelas, A. Gómez, A. Bahsas, J. Amaro-Luis, Arch. Pharm. Chem. Life Sci. 338, 32, (2005).
- L. Hu, H. M. Martin, T. J. Strathmann, Environ. Sci. Technol. 44, 6416, (2010).
- W. M Shi, X. P. Ma, G. F. Su, D. L. Mo, Org. Chem. Front. 3, 116, (2016).
- I. Brito, J. Bórquez, D. Robledo, M. J. Simirgiotis, A. Cárdenas, J. Chil. Chem. Soc. 63, 4086, (2018).
- A. M. Maharramov, G. Sh. Duruskari, G. Z. Mammadova, A. N. Khalilov, J. M. Aslanova, J. Cisterna, A. Cárdenas, I. Brito, J. Chil. Chem. Soc. 64, 4441, (2019).
- A. R. Asgarova, A. N. Khalilov, I. Brito, A. M. Maharramov, N. G. Shikhaliyev, J. Cisterna, A. Cárdenas, A. V. Gurbanov, F. I. Zubkov, K. T. Mahmudov, Acta Cryst. C75, 342, (2019).
- G. Mahmoudia, S. Rostamnia, G. Zaragoza, I. Brito, J. Cisterna, A. Cárdenas, J. Chil. Chem. Soc. (in press).
- G. E. Delgado, E. Osal, A. J. Mora, T. González, A. Palma, A. Bahsas, J. Struct. Chem. 59, 1248, (2018).
- G. E. Delgado, J. A. Henao, J. H. Quintana, H. M. Al-Maqtari, J. Jamalis, H. M. Sirat, J. Struct. Chem. 59, 1493, (2018).
- L. E. Fernández, G. E. Delgado, L. V. Maturano, R. M. Tótaro, E. L. Varetti, J. Mol. Struct. 1168, 84, (2018).
- G. E. Delgado, S. M. Liew, J. Jamalis, J. Cisterna, A. Cárdenas, I. Brito, J. Mol. Struct. 1210, 128044, (2020).
- M. A. Spackman, P. G. Byrom, Chem. Phys. Letters 267, 215, (1997).
- M. J. Turner, S. P. Thomas, M. W. Shi, D. Jayatilaka, M. A. Spackman, Chem. Commun. 51, 3735, (2015).
- G. M. Sheldrick, Acta Cryst. A. 64, 112, (2008).
- G. M. Sheldrick, Acta Cryst. C. 71, 3, (2015).
- A. L. Spek, J. Appl. Cryst. 36, 7, (2003).
- M. J. Turner, J. J. McKinnon, S. K. Wolff, S. K. Grimwood, P. R. Spackman, D. Jayatilaka, M. A. Spackman, Crystal Explorer 17.5. University of Western Australia, 2017.
- M. A. Spackman, J. J. McKinnon, CrystEngComm 4, 378, (2002).
- C. R. Groom, F. H. Allen, Angew. Chem. Int. Ed. 53, 662, (2014).
- S. E. Denmark, J. I. Montgomery, J. Org. Chem. 71, 6211, (2006).
- P. Merino, V. Mannucci, T. Tejero, Eur. J. Org. Chem. 23, 3943, (2008).
- A. V. Churakov, P. V. Prikhodchenko, A. G. Medvedev, A. A. Mikhaylov, Acta Cryst. E, 73, 1666 (2017).
- M. C. Etter, Acc. Chem. Res. 23, 120, (1990).
- A. Le Bail, Powder Diffr. 20, 316, (2005)
- J. Rodríguez-Carvajal, Fullprof version 7.20, Laboratoire Léon Brillouin (CEA-CNRS), France, 2019.