
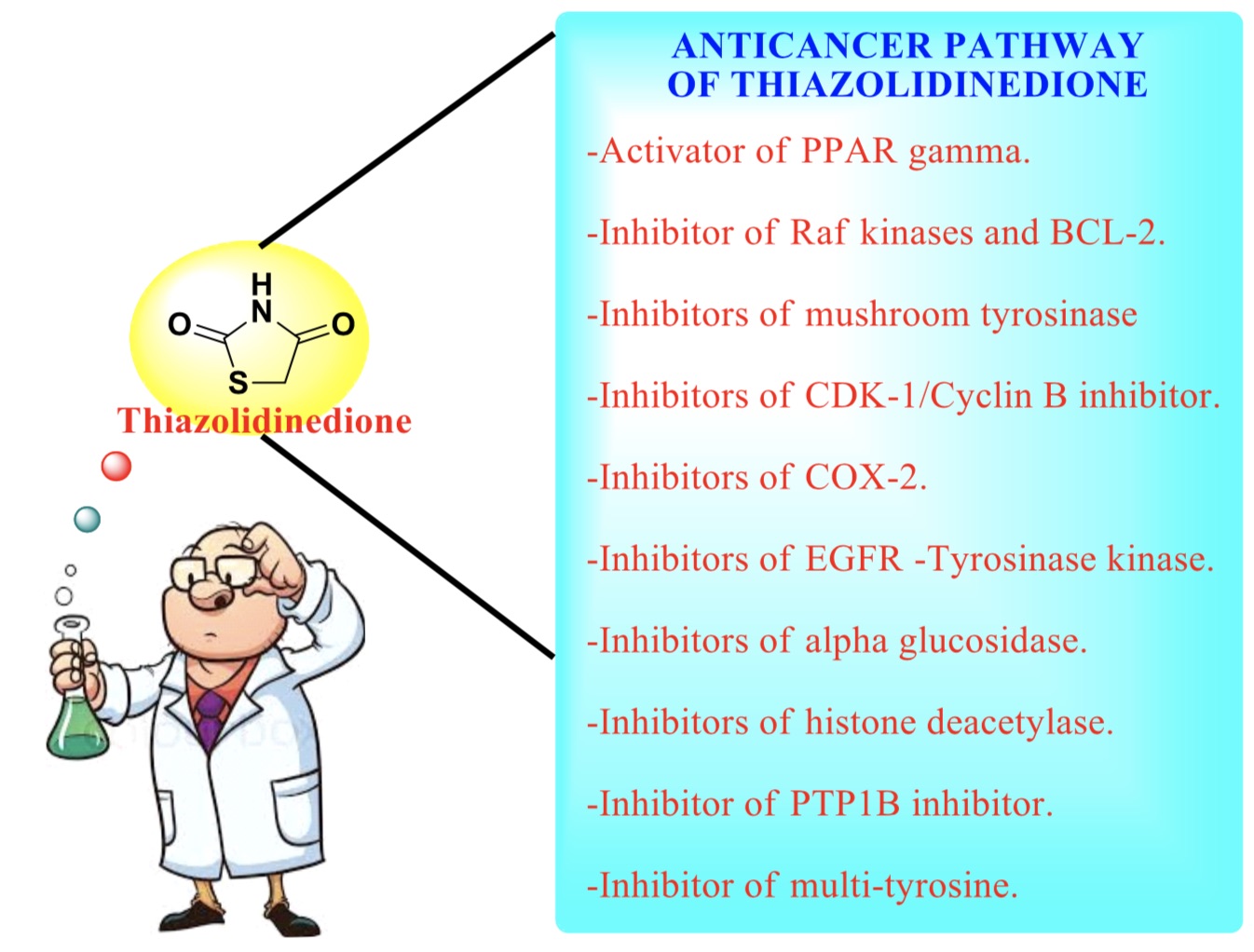
- TZD,
- SAR,
- PPARγ,
- HDAC inhibitors,
- AGIs
- PTP1B inhibitor,
- EGFR and Mushroom Tyrosine kinase inhibitor,
- COX enzyme inhibitors ...More
Copyright (c) 2020 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
In current review, authors aim to inspire the researcher through structure activity relationship strategy for the finding of safe and effective anticancer molecules. Nowadays cancer is measured as one of the major health problems in human beings in the world from decades. A classes of heterocyclic compounds have been recognized through molecular biology, empirical screening and rational drug development for the evaluation of anticancer molecules however regrettably, till now we could not find a medicine to be entirely active and nontoxic for the treatment of cancer patients. In pointed view, it might be measured that Thiazolidinedione (TZD) heterocyclic compounds are prodigious standing in the synthetic and pharmacological approach of medicinal chemistry. Thiazolidinedione (TZD) nucleus upon the substitution of various functional groups is provides a wide spectrum of biological activity by the use of different mechanism on different target sites. Recently, some of the substituted thiazolidinedione molecules are designed for the treatment of human cancers cell line through different molecular mechanism such as EGFR & Mushroom Tyrosine kinase inhibitor, COX enzyme inhibitors, Histone deacetylase inhibitors, Alpha glucosidase inhibitor, DNA intercalation and Protein tyrosine phosphatase 1B (PTP1B) inhibitor, basically in which PPAR gamma express are in high levels. Peroxisome proliferator-activated receptor (PPAR) gamma ligands effect on apoptosis, cell proliferation and cell differentiation on different types of cell. The most commonly cascades in human cancers cell are Raf/MEK/ERK, Wnt and PI3/Akt. This article highlights and embraces a concise overview of recent approaches for the synthesis of new thiazolidinedione molecules with its structure activity relationship strategy and effects on various signaling pathways, which is responsible for the expresses of cancer cell line activity.
References
- A.Z. Mirza, I.I. Althagafi, H. Shamshad, Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications, Eur. J. Med. Chem. 15 (2019) 502-513.
- A. Husain, M. Rashid, R. Mishra, S. Parveen, S.D. Soo, D. Kumar, Benzimidazole bearing oxadiazole and triazolo-thiadiazoles nucleus: Design and synthesis as anticancer agents, Bioorg. Med. Chem. Lett. 22 (2012) 5438–5444.
- M. Rashid, A. Husain, R. Mishra, Synthesis of benzimidazoles bearing oxadiazole nucleus as anticancer agents, Eur. J. Med. Chem. 54 (2012) 855-866.
- A.N. Bhatt, R. Mathur, A. Farooque, Cancer biomarkers - current perspectives, Indian J. Med. Res. 132 (2010) 129-49.
- L. Rebecca, M.P.H. Siegel, D. Kimberly, M.P.H. Miller, D.V.M. Ahmedin Jemal, Cancer statistics-2019, American Cancer Society Journals, 69 (2019) 7-34.
- R.A. Smith, V. Cokkinides, O.W. Brawley, Cancer screening in the United States, Cancer J. Clin. 59 (2009) 27-41.
- G.A. Duenas, L.P. Garcıa, L.A. Herrera, The prince and the pauper. A tale of anticancer targeted agents, Mol. Cancer. 7 (2008) 82.
- M.V. Nora de Souza, Synthesis and biological activity of natural thiazoles: An important class of heterocyclic compounds, J. Sulfur Chem. 26 (2005) 429-449.
- A. Nefzi, J.M. Ostresh, R.A. Houghten, The Current Status of Heterocyclic Combinatorial Libraries, Chem. Rev. 97 (1997) 449.
- M. Yu, M. Tsuyoshi, Y. Tohru, K. Mitsuru, O. Hiroyuki, I. Hitoshi, S. Takashi, Novel 5-substituted 2,4-thiazolidinedione and 2,4-oxazolidinedione derivatives as insulin sensitizers and anti-diabetic activities, J. Med. Chem. 45 (2002) 1518-1534.
- A. Galli, T. Mello, E. Ceni, E. Surrenti, C. Surrenti, The potential of antidiabetic thiazolidinediones for anticancer therapy, Expert Opin. Investig. Drugs. 15(9) (2006) 1039-1049.
- V. Asati, D.K. Mahapatra, S.K. Bharti, Thiazolidine-2,4-diones as multi-targeted scaffold in medicinal chemistry: Potential anticancer agents, Eur. J. Med. Chem. 24(87) (2014) 814-33.
- P.G. Jain, B.D. Patel, Medicinal chemistry approaches of poly ADP-Ribose polymerase 1 (PARP1) inhibitors as anticancer agents-A recent update, Eur. J. Med. Chem. 1(165) (2019) 198-215.
- D. Panigrahy, L.Q. Shen, M.W. Kieran, A. Kaipainen, Therapeutic potential of thiazolidinediones as anticancer agents, Expert Opin. Investig. Drugs. 12(12) (2003) 1925-37.
- J.P. Ye, Challenges in drug discovery for thiazolidinedione substitute, Acta. Pharm. Sin. B. 1 (2011) 137-142.
- C.V. Rizos, M.S. Elisaf, D.P. Mikhailidis, E.N. Liberopoulos, How safe is the use of thiazolidinediones in clinical practice?, Expert Opin. Drug Saf. 8(1) (2009) 15-32.
- J.N. Feige, L. Gelman, L. Michalik, B. Desvergne, W. Wahli, From molecular action to physiological outputs: Peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Progress in Lipid Research, 45(2) (2006) 120–159.
- Ferlay J, Shin HR, Bray F, Forman D, Mathers CD, Parkin D. GLOBOCAN 2008, Cancer Incidence and Mortality, World Health Organization, The Global Burden of Disease: 2004 Update. Geneva: World Health Organization; 2008.
- Worldwide: IARC Cancer Base No. 10. Lyon, France: International Agency for Research on Cancer; Year. Available at: http://globocan.iarc.fr. 2010. Last accessed 8/17/2010.
- C. Juge-Aubry, A. Pernin, T. Favez , DNA binding properties of peroxisome proliferator-activated receptor subtypes on various natural peroxisome proliferator response elements. Importance of the 5'-flanking region, The J. Bio. Chem. 272 (40) (1997) 25252–25259.
- J. Di-Renzo, M. Soderstrom, R. Kurokawa, Peroxisome proliferator-activated receptors and retinoic acid receptors differentially control the interactions of retinoid X receptor heterodimers with ligands, coactivators, and corepressors, Molecular and Cellular Biology, 17 (4) (1997) 2166–2176.
- E.M. Mc-Inerney, D.W. Rose, S.E. Flynn, Determinants of coactivator LXXLL motif specificity in nuclear receptor transcriptional activation, Genes & Development, 12(21) (1998) 3357–3368.
- C.X. Yuan, M. Ito, J.D. Fondell, Z.Y. Fu, and R.G. Roeder, The TRAP220 component of a thyroid hormone receptor-associated protein (TRAP) coactivator complex interacts directly with nuclear receptors in a ligand-dependent fashion, Proceedings of the National Academy of Sciences of the United States of America, 95(14) (1998) 7939–7944.
- R. Lesyk, B. Zimenkovsky, D. Atamanyuk, F. Jensen, K. Kiec-Kononowicz, A. Gzella, Anticancer thiopyrano [2,3-d][1,3]thiazol-2-ones with norbornane moiety.Synthesis, cytotoxicity, physico-chemical properties and computational studies, Bioorg. Med. Chem. 14(15) (2006) 5230-40.
- A.G. Chittiboyina, M.S. Venkatraman, C.S. Mizuno, P.V. Desai, A. Patny, S.C. Benson, M.A. Avery, Design and Synthesis of the First Generation of Dithiolane Thiazolidinedione- and Phenylacetic Acid-Based PPARγ Agonists, J. Med. Chem. 49(14) (2006) 4072–4084.
- R.F. George, Stereoselective synthesis and QSAR study of cytotoxic 2-(4-oxo-thiazolidin-2-ylidene)-2-cyano-N-arylacetamides, Eur. J. Med. Chem. 47(1) (2012) 377-86.
- V.R. Avupati, R.P. Yejella, A. Akula, G.S. Guntuku, B.R. Doddi, V.R. Vutla, S.R. Anagani, L.S. Adimulam, A.K. Vyricharla, Synthesis, characterization and biological evaluation of some novel 2,4-thiazolidinediones as potential cytotoxic, antimicrobial and antihyperglycemic agents, Bioorg. Med. Chem. Lett. 22(20) (2012) 6442-50.
- R. Romagnoli, P.G. Baraldi, M.K. Salvador, M.E. Camacho, J. Balzarini, J. Bermejo, F. Estevez, Anticancer activity of novel hybrid molecules containing 5-benzylidene thiazolidine-2,4-dione, Eur. J. Med. Chem. 63 (2013) 544-57.
- G. Odabaei, D. Chatterjee, A.R. Jazirehi, L. Goodglick, K. Yeung, B. Bonavida, Raf-1 Kinase Inhibitor Protein: Structure, Function, Regulation of Cell Signaling, and Pivotal Role in Apoptosis, Advances in Can. Res. 91 (2004) 169–200.
- K. Lorenz, M.J. Lohse, U. Quitterer, Protein kinase C switches the Raf kinase inhibitor from Raf-1 to GRK-2, Nature. 426 (2003) 574-579.
- D. Strumberg, S. Seeber, Raf kinase inhibitors in oncology, Onkologie. 28(2) (2005) 101-7.
- E.T. Keller, Z. Fu, M. Brennan, The role of Raf kinase inhibitor protein (RKIP) in health and disease. Biochem. Pharmacol, 68(6) (2004) 1049-53.
- D. Havrylyuk, L. Mosula, B. Zimenkovsky, O. Vasylenko, A. Gzella, R. Lesyk, Synthesis and anticancer activity evaluation of 4-thiazolidinones containing benzothiazole moiety, Eur. J. Med. Chem. 45(11) (2010) 5012-21.
- K. Liu, W. Rao, H. Parikh, Q. Li, T.L. Guo, S. Grant, G.E. Kellogg, S. Zhang, 3,5-Disubstituted-thiazolidine-2,4-dione analogs as anticancer agents: design, synthesis and biological characterization, Eur. J. Med. Chem. 47(1) (2012) 125-37.
- M.J. Rego, M. Galdino-Pitta, M.R. Pereira, D.T.M. da Silva, J.C. Rabello, M.M. Alves de Lima, M. do , M.G. da Rocha Pitta, Synthesis, in vitro anticancer activity and in silico study of new disubstituted thiazolidinedione derivatives. Med. Chem. Res., 23(6) (2014) 3220–3226.
- P. Sharma, T.S. Reddy, N.P. Kumar, K.R. Senwar, S.K. Bhargava, N. Shankaraiah, Conventional and microwave-assisted synthesis of new 1H-benzimidazole-thiazolidinedione derivatives: A potential anticancer scaffold, Eur. J. Med. Chem. 138 (2017) 234-245.
- P. Sharma, T. Srinivasa Reddy, D. Thummuri, K.R. Senwar, N. Praveen Kumar, V.G.M. Naidu, S.K. Bhargava, N. Shankaraiah, Synthesis and biological evaluation of new benzimidazole-thiazolidinedione hybrids as potential cytotoxic and apoptosis inducing agents, Eur. J. Med. Chem. 124 (2016) 608-621.
- A.H. Abdelazeem, M.T. El-Saadi, E.G. Said, B.G.M. Youssif, H.A. Omar, S.M. El-Moghazy, Novel diphenylthiazole derivatives with multi-target mechanism: Synthesis, docking study, anticancer and anti-inflammatory activities, Bioorg. Chem. 75 (2017) 127-138.
- B. Qi, Y.Yang, H. He, X. Yue, Y. Zhou, X. Zhou, Y. Chen, M. Liu, A. Zhang, F. Wei, Identification of novel N(1)-(2-aryl-1, 3-thiazolidin-4-one)-N(3)-aryl ureasshowing potent multi-tyrosine kinase inhibitory activities, Eur. J. Med. Chem. (146) (2018) 368-380.
- B. Qi, Y. Yang, G. Gong, H. He, X. Yue, X. Xu, Y. Hu, J. Li, T. Chen, X. Wan, A. Zhang, G. Zhou, Discovery of N1-(4-((7-(3-(4-ethylpiperazin-1-yl)propoxy)-6-methoxyquinolin-4- yl)oxy)-3,5-difluorophenyl)-N3-(2-(2,6-difluorophenyl)-4-oxothiazolidin-3-yl)urea as a multi-tyrosine kinase inhibitor for drug-sensitive and drug-resistant cancers treatment, Eur. J. Med. Chem. 23 (2018) 432-448.
- https://www.cancer.gov/publications/cancer-terms/def/egfr-tyrosine-kinase-inhibitor.
- Y. Yarden, J. Schlessinger, Epidermal growth factor induces rapid, reversible aggregation of the purified epidermal growth factor receptor, Biochemistry. 26 (1987) 1443–1451.
- J. Downward, P. Parker, M.D. Waterfield, Autophosphorylation sites on the epidermal growth factor receptor. Nature. 311 (1984) 483–485.
- K. Oda, Y. Matsuoka, A. Funahashi, H. Kitano, A comprehensive pathway map of epidermal growth factor receptor signaling, Mol. Syst. Biol. 1 (2005) 10-21.
- P.C. Lv, C.F. Zhou, J. Chen, P.G. Liu, K.R. Wang, W.J. Mao, H.Q. Li, Y. Yang, J. Xiong, H.L. Zhu, Design, synthesis and biological evaluation of thiazolidinone derivatives as potential EGFR and HER-2 kinase inhibitors, Bioorg. Med. Chem. 18(1) (2010) 314-9.
- K.M. Qiu, H.H. Wang, L.M. Wang, Y. Luo, X.H. Yang, X.M. Wang, H.L. Zhu, Design, synthesis and biological evaluation of pyrazolyl-thiazolinone derivatives as potential EGFR and HER-2 kinase inhibitors, Bioorg. Med. Chem. 20(6) (2012) 2010-8.
- Y.S. Sung, K.S. Vinay, N. Sharma, Mushroom Tyrosinase: Recent Prospects, J. of Agricultural and Food Chemistry 2003, 51(10), 2837-2853.
- I. Kubo, Y. Yokokawa, Two tyrosinase inhibiting flavonol glycosides from Buddleia coriacea, Phytochemistry. 31 (1992) 1075-1077.
- I. Kubo, Y. Yokokawa, I. Kinst-Hori, Tyrosinase inhibitors from Bolivian medicinal plants, J. Nat. Prod. 58 (1995) 739-743.
- J.K. No, D.Y. Soung, Y.J. Kim, K.H. Shim, Y.S. Jun, S.H. Rhee, T. Yokozawa, H.Y. Chung, Inhibition of tyrosinase by green tea components, Life Sci. 65 (1999) 241-246.
- S.E. Lee, M.K. Kim, S.G. Lee, Y.J. Ahn, H.S. Lee, Inhibitory effects of Cinnamomum cassia bark-derived materials on mushroom tyrosinase, Food Sci. Biotechnol. 9 (2000) 330-333.
- I. Kubo, I. Kinst-Hori, Tyrosinase inhibitors from anise oil, J. Agric. Food Chem. 46 (1998) 1268-1271.
- I. Kubo, I. Kinst-Hori, Tyrosinase inhibitors from cumin, J. Agric. Food Chem. 46 (1988) 5338-5341.
- H.S. Lee, Tyrosinase inhibitors of Pulsatilla cernua root-derived materials, J. Agric. Food Chem. 50 (2002) 1400-1403.
- Y.M. Ha, Y.J. Park, J.A. Kim, D. Park, J.Y. Park, H.J. Lee, J.Y. Lee, H.R. Moon, H.Y. Chung, Design and synthesis of 5-(substituted benzylidene) thiazolidine-2,4-dione derivatives as novel tyrosinase inhibitors, Eur. J. Med. Chem. 49 (2012) 245-52.
- A. Zarghi, S. Arfaei, Selective COX-2 Inhibitors: A Review of their Structure-Activity Relationships, Iran J. Pharm. Res. 10(4) (2011) 655-83.
- N. Hashemi Goradel, M. Najafi, E. Salehi, B. Farhood, K. Mortezaee, Cyclooxygenase-2 in cancer: A review, J. Cell Physiol. 234(5) (2019) 5683-5699.
- A.H. Abdelazeem, A.M. Gouda, H.A. Omar, M.F. Tolba, Design, synthesis and biological evaluation of novel diphenylthiazole-based cyclooxygenase inhibitors as potential anticancer agents, Bioorg. Chem. 57 (2014) 132-41.
- A.M. Shawky, M.A.S. Abourehab, A.N. Abdalla, A.M. Gouda, Optimization of pyrrolizine-based Schiff bases with 4-thiazolidinone motif: Design, synthesis and investigation of cytotoxicity and anti-inflammatory potency, Eur. J. Med. Chem. 185 (2020) 1157-80.
- H.J. Kim, S.C. Bae, Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anti-cancer drugs, Am. J. Transl. Res. 3(2) (2011) 166-79.
- J.H. Lee, M.L. Choy, L. Ngo, S.S. Foster, P.A. Marks, Histone deacetylase inhibitor induces DNA damage, which normal but not transformed cells can repair. Proceedings of the National Academy of Sciences, 107(33) (2010) 14639-14644.
- O. Katoch, B. Dwarakanath, P.K. Agrawala, HDAC inhibitors: applications in oncology and beyond. HOAJ Biology, 2 (2013) 21-34.
- R. Mohan, A.K. Sharma, S. Gupta, C.S. Ramaa, Design, synthesis, and biological evaluation of novel 2,4-thiazolidinedione derivatives as histone deacetylase inhibitors targeting liver cancer cell line. Med. Chem. Res. 21(7) (2011) 1156–1165.
- J.S. Venable, D.S. Aschenbrenner, Drug Therapy in Nursing, Hagerstown, MD: Lippincott Williams & Wilkins (2006).
- J.B. Popovic-Djordjevic, I.I. Jevtic, N.D. Grozdanic, S.B. Segan, M.V. Zlatovic, M.D. Ivanovic, T.P. Stanojkovic, α-Glucosidase inhibitory activity and cytotoxic effects of some cyclic urea and carbamate derivatives, J. Enzyme Inhib. Med. Chem. 32(1) (2017) 298-303.
- Y. Chinthala, A. Kumar Domatti, A. Sarfaraz, S.P. Singh, N. Kumar Arigari, N. Gupta, S.K. Satya, J. Kotesh Kumar, F. Khan, A.K. Tiwari, G. Paramjit, Synthesis, biologicalevaluation and molecular modeling studies of some novel thiazolidinediones with triazole ring, Eur. J. Med. Chem. 70 (2013) 308-14.
- L.H. Hurley, DNA and its associated processes as targets for cancer therapy, Nat. Rev. Cancer. 2(3) (2002) 188-200.
- R. Palchaudhuri, P.J. Hergenrother, DNA as a target for anticancer compounds: methods to determine the mode of binding and the mechanism of action, Curr. Opin. Biotechnol. 18(6) (2007) 497-503.
- A.S. Biebricher, I. Heller, R.F. Roijmans, T.P. Hoekstra, E.J. Peterman, G.J. Wuite, The impact of DNA intercalators on DNA and DNA-processing enzymes elucidated through force-dependent binding kinetics, Nat. Commun. 18 (6) (2015) 7304-15.
- D.A. Koster, K. Palle, E.S. Bot, M.A. Bjornsti, N.H. Dekker, Antitumour drugs impede DNA uncoiling by topoisomerase I. Nature. 448 (7150) (2007) 213-7.
- A. Mukherjee, R. Lavery, B. Bagchi, J.T. Hynes, On the molecular mechanism of drug intercalation into DNA: a simulation study of the intercalation pathway, free energy and DNA structural changes, J. Am. Chem. Soc. 130(30) (2008) 9747-55.
- N. Shankaraiah, R. Tokala, S. Thatikonda, S. Sana, P. Regur, C. Godugu, Synthesis and in vitro cytotoxicity evaluation of β-carboline-linked 2,4-thiazolidinedione hybrids: Potential DNA intercalation and apoptosis inducing studies. New Journal of Chemistry, 23 (2018) 214-231
- J. Chernoff , A.R. Schievella, C.A. Jost, R.L.Erikson, B.G. Neel, Cloning of a cDNA for a major human protein-tyrosine-phosphatase. Proc. Natl. Acad. Sci. U.S.A. 87(7) (1990) 2735–9.
- J.V. Frangioni, P.H. Beahm, V. Shifrin, C.A. Jost, B.G. Neel, The non transmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence, Cell. 68 (3) (1992) 545–60.
- A. Alonso, J. Sasin, N. Bottini, I. Friedberg, I. Friedberg, A. Osterman, A. Godzik, T. Hunter, J. Dixon, T. Mustelin, Protein tyrosine phosphatases in the human genome, Cell. 117 (6) (2004) 699–711.
- F. Liu, D.E. Hill, J. Chernoff, Direct binding of the proline-rich region of protein tyrosine phosphatase 1B to the Src homology 3 domain of p130 (Cas), J. Biol. Chem. 271 (49) (1996) 31290–5.
- N.K. Tonks, PTP1B: from the sidelines to the front lines, FEBS Letters. 546 (1) (2003) 140–148.
- A. Tahseen, S. Bharti, R.K. Abdul, K. Atul, Design and synthesis of benzylidene thiazolidine-2,4-dione derivative with their anticancer and antidiabetic biological screening, World J. Pharma. Res. 3(6) (2014) 143-9.
- D. Kaminskyy, B. Bednarczyk-Cwynar, O. Vasylenko, O. Kazakova, B. Zimenkovsky, L. Zaprutko, R. Lesyk, Synthesis of new potential anticancer agents based on 4-thiazolidinone and oleanane scaffolds. Med. Chem. Res. 21(11) (2011) 3568–3580.
- K. Sudheer, B.R. Madhava, V. Harinadha Babu, Synthesis of some novel 2,4-thiazolidinedione incorporated pyrazole derivatives as anticancer agents, Chemistry, 6(2) (2014) 1-9.
- T. Anh Hle, N.T. Cuc, B.H. Tai, P.H. Yen, N.X. Nhiem, T. Thao, N.H. Nam, C. Van Minh, P. Van Kiem, Y.H. Kim, Synthesis of chromonylthiazolidines and their cytotoxicity to human cancer cell lines, Molecules. 20(1) (2015) 1151-60.
- J. Senkiv, N. Finiuk, D. Kaminskyy, D. Havrylyuk, M. Wojtyra, L. Kril, R. Lesyk, 5-Ene-4-thiazolidinones induce apoptosis in mammalian leukemia cells. Eur. J. Med. Chem. 117 (2016) 33–46.
- C. Ozen, M. Ceylan Unlusoy, N. Aliary, M. Ozturk, O. Bozdag Dundar, Thiazolidinedione or Rhodanine: A Study on Synthesis and Anticancer Activity Comparison of Novel Thiazole Derivatives, J. Pharm. Sci. 20(1) (2017) 415-427.
- K. Metwally, H. Pratsinis, D. Kletsas, Novel 2,4- thiazolidinediones: Synthesis, in vitro cytotoxic activity, and mechanistic investigation, Eur. J. Med. Chem. 133 (2017) 340–350.
- D.M. Corigliano, R. Syed, S. Messineo, A. Lupia, R. Patel, C.V.R. Reddy, A. Brunetti, Indole and 2,4-Thiazolidinedione conjugates as potential anticancer modulators, Peer J. 6 (2018) 386.

