
- quinoline,
- antibacterial activity,
- fluorescence quenching,
- broth microdilution
Copyright (c) 2020 Journal of the Chilean Chemical Society

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
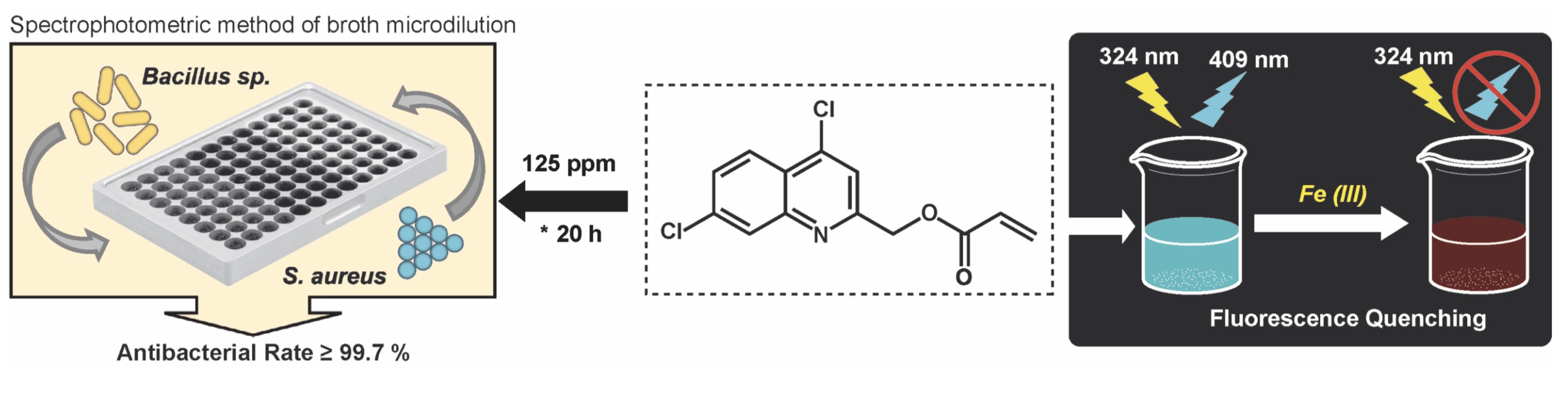
Currently, some quinoline-based anticancer drugs are successful repurposed for treatment of bacterial infections. This study assessed the antibacterial activity of the new anticancer compound 4,7-dichloro-2-quinolinemethylacrylate (AQM) against bacteria of both clinical and agricultural interest, and also determined the influence of some metal cations (Fe3+, Mn2+, Zn2+, Na+, Mg2+, Co2+ and Ni2+) on the AQM photophysics. The synthesis of AQM was carried out by the reported method. The antibacterial activity of AQM on Pectobacterium carotovorum subsp. carotovorum (Pcc), Ralstonia solanacearum, Klebsiella pneuminiae, Pseudomonas syringae, Staphylococcus aureus y Bacillus sp., was evaluated using the spectrophotometric method of broth microdilution. At 125 ppm, AQM produced growth inhibition of 51.7% in Pcc, and a bacteriostatic effect in S. aureus and Bacillus sp., but no effect was seen against the other bacteria. In addition, fluorescence quenching of AQM solution induced only by Fe3+ ion, and not by the other metal cations, was confirmed. These results postulate that AQM could be a molecule with potential application in antibacterial therapy or in the fluorometric detection of Fe3+ ions.
References
- S. M. A. Hussaini, Expert Opin. Ther. Pat. 26, 1201 (2016)
- A. Marella, O. P. Tanwar, R. Saha, M. R. Ali, S. Srivastava, M. Akhter, M. Shaquiquzzaman, and M. M. Alam, Saudi Pharm. J. 21, 1 (2013)
- C.-H. Lee and H.-S. Lee, J. Korean Soc. Appl. Biol. Chem. 52, 331 (2009)
- M. Artico, A. Mai, G. Sbardella, S. Massa, C. Musiu, S. Lostia, F.
- Demontis, and P. La Colla, Bioorganic Med. Chem. Lett. 9, 1651 (1999)
- G. S. Bisacchi, J. Med. Chem. 58, 4874 (2015)
- R. Cherdtrakulkiat, S. Boonpangrak, N. Sinthupoom, S. Prachayasittikul, S. Ruchirawat, and V. Prachayasittikul, Biochem. Biophys. Reports 6, 135 (2016)
- V. Prachayasittikul, V. Prachayasittikul, S. Prachayasittikul, and S. Ruchirawat, Drug Des. Devel. Ther. 7, 1157 (2013)
- M. Asif, Ann Med Chem Res 1, 1003 (2014)
- M. A. Kohanski, D. J. Dwyer, and J. J. Collins, Nat. Rev. Microbiol. 8, 423 (2010)
- V. Uivarosi, Molecules 18, 11153 (2013)
- C. Pelletier, P. Prognon, and P. Bourlioux, Antimicrob. Agents Chemother. 39, 707 (1995)
- H. Huber-Emden, A. Hubele, and G. Klahre, Patent US3873703A, 1975
- D. Ghisalberti, A. Mahamoud, J. Chevalier, M. Baitiche, M. Martino, J.-M. Pagès, and J. Barbe, Int. J. Antimicrob. Agents 27, 565 (2006)
- J.-M. Rolain, P. Colson, and D. Raoult, Int. J. Antimicrob. Agents 30, 297 (2007)
- A. So, B. A. Nacev, C. R. Chong, H. C. Bhang, J. Xu, J. S. Shim, J. O. Liu, K. C. Han, M. G. Pomper, S. Bhat, S. Dhara, and Y. Matsui, JNCI J. Natl. Cancer Inst. 102, 1855 (2010)
- B. Mirković, B. Markelc, M. Butinar, A. Mitrović, I. Sosič, S. Gobec, O. Vasiljeva, B. Turk, M. Čemažar, G. Serša, and J. Kos, Oncotarget 6, 19027 (2015)
- J. Lazovic, L. Guo, J. Nakashima, L. Mirsadraei, W. Yong, H. J. Kim, B. Ellingson, H. Wu, and W. B. Pope, Neuro. Oncol. 17, 53 (2014)
- W.-L. Chang, L.-C. Hsu, W.-J. Leu, C.-S. Chen, and J.-H. Guh, Oncotarget 6, 39806 (2015)
- V. Prachayasittikul, W. Chan-On, H. Nguyen Thi Bich, N. Songtawee, W. Suwanjang, and S. Prachayasittikul, Drug Des. Devel. Ther. 9, 2033 (2015)
- H. Jiang, J. E. Taggart, X. Zhang, D. M. Benbrook, S. E. Lind, and W.-Q. Ding, Cancer Lett. 312, 11 (2011)
- P. Huang and Q. Li, Patent WO 2017/173278 A1, 2017
- M. Fiorillo, R. Lamb, H. B. Tanowitz, A. R. Cappello, U. E. Martinez-Outschoorn, F. Sotgia, and M. P. Lisanti, Aging (Albany. NY). 8, 1593 (2016)
- P. Pantziarka, V. Sukhatme, L. Meheus, V. Sukhatme, and G. Bouche, BioRxiv 197434 (2017)
- Q. Dong, J. Luo, W. Qiu, L. Cai, S. Anjum, B. Li, M. Hou, G. Xie, G. Sun, Q. Dong, J. Luo, W. Qiu, L. Cai, S. I. Anjum, B. Li, M. Hou, G. Xie, and G. Sun, Molecules 21, 978 (2016)
- H. Valle, R. Palao-Suay, M. R. Aguilar, J. S. Román, J. Becerra, B. Rivas, and R. V. Mangalaraja, J. Appl. Polym. Sci. 136, 47545 (2019)
- CLSI, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically (CLSI Document M07-A9), Approved Standard – 9th Ed. (Clinical and Laboratory Standards Institute, Wayne, Pennsylvania, 2012), pp. 16–20
- S. Singh, K. K. Roy, S. R. Khan, V. K. Kashyap, A. Sharma, S. Jaiswal, S. K. Sharma, M. Y. Krishnan, V. Chaturvedi, J. Lal, S. Sinha, A. Dasgupta, R. Srivastava, and A. K. Saxena, Bioorg. Med. Chem. 23, 742 (2015)
- I. J. Pachter, J. Am. Chem. Soc. 75, 3026 (1953)
- A. Esparza-Ruiz, C. Herrmann, J. Chen, B. O. Patrick, E. Polishchuk, and C. Orvig, Inorganica Chim. Acta 393, 276 (2012)
- B. Valeur and M. N. Berberan-Santos, Molecular Fluorescence : Principles and Applications, 2nd Ed. (Wiley-VCH, Weinheim, 2012), pp. 72–74
- D. S. Karpovich and G. J. Blanchard, J. Phys. Chem. 99, 3951 (1995)
- Y. W. Choi, G. J. Park, Y. J. Na, H. Y. Jo, S. A. Lee, G. R. You, and C. Kim, Sensors Actuators B Chem. 194, 343 (2014)
- X. Bao, X. Cao, X. Nie, Y. Xu, W. Guo, B. Zhou, L. Zhang, H. Liao, and T. Pang, Sensors Actuators B Chem. 208, 54 (2015)
- P. Kaur, H. Kaur, and K. Singh, RSC Adv. 3, 64 (2013)
- X. Yang, P. Zhao, J. Qu, and R. Liu, Luminescence 30, 592 (2015)
- Z. A. Machan, G. W. Taylor, T. L. Pitt, P. J. Cole, and R. Wilson, J. Antimicrob. Chemother. 30, 615 (1992)
- F. Salvaggio, J. T. Hodgkinson, L. Carro, S. M. Geddis, W. R. J. D. Galloway, M. Welch, and D. R. Spring, European J. Org. Chem. 434 (2016)
- P. A. Lambert, J. R. Soc. Med. 95 Suppl 4, 22 (2002)
- J. Ruiz, J. Antimicrob. Chemother. 51, 1109 (2003)
- R. Clinton and C. Suter, Patent US2505462A, 1950
- M. Rudrapal and D. Chetia, Int. J. ChemTech Res. 2, 1606 (2010)
- R. Jagadeesh, K. N. Saivisveswar, and S. P. Revankar, Sch. J. App. Med. Sci. 2, 3046 (2014)
- M. V. N. de Souza, K. C. Pais, C. R. Kaiser, M. A. Peralta, M. de L. Ferreira, and M. C. S. Lourenço, Bioorg. Med. Chem. 17, 1474 (2009)
- M. Toyofuku, T. Nakajima-Kambe, H. Uchiyama, and N. Nomura, Microbes Environ. 25, 1 (2010)
- S. Heeb, M. P. Fletcher, S. R. Chhabra, S. P. Diggle, P. Williams, and M. Cámara, FEMS Microbiol. Rev. 35, 247 (2011)
- H. Budzikiewicz, Siderophores of the Pseudomonadaceae sensu stricto (Fluorescent and Non-Fluorescent Pseudomonas spp.), in Progress in the Chemistry of Organic Natural Products, ed. by W. Herz, H. Falk, and G. W. Kirby (Springer Vienna, Vienna, 2004), p. 81
- S. P. Diggle, S. Matthijs, V. J. Wright, M. P. Fletcher, S. R. Chhabra, I. L. Lamont, X. Kong, R. C. Hider, P. Cornelis, M. Cámara, and P. Williams, Chem. Biol. 14, 87 (2007)
- P. W. Royt, R. V. Honeychuck, V. Ravich, P. Ponnaluri, L. K. Pannell, J. S. Buyer, V. Chandhoke, W. M. Stalick, L. C. DeSesso, S. Donohue, R. Ghei, J. D. Relyea, and R. Ruiz, Bioorg. Chem. 29, 387 (2001)
- G. Palmer, J. Schertzer, L. Mashburn-Warren, M. Whiteley, Methods Mol. Biol. 692, 207 (2011)
- W. Raza, W. Hongsheng, and S. Qirong, Brazilian Arch. Biol. Technol. 53, 1145 (2010)
- F. Imperi, K. A. Mettrick, M. Shirley, F. Tiburzi, R. C. Draper, P. Visca, and I. L. Lamont, Iron Transport and Signaling in Pseudomonads, in Pseudomonas, ed. by B. H. Rehm (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2008), p. 129
- J. S. Brown and D. W. Holden, Microbes Infect. 4, 1149 (2002)

