ASSESSMENT OF APPLE AND CARROT POMACES FOR COST-EFFECTIVE REACTIVE BLACK 5 BIOREMOVAL BY PENICILLIUM CITRINUM
- Apple pomace,
- bioremoval,
- carrot pomace,
- Penicillium citrinum,
- Reactive Black
Copyright (c) 2020 Ekin Demiray

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Abstract
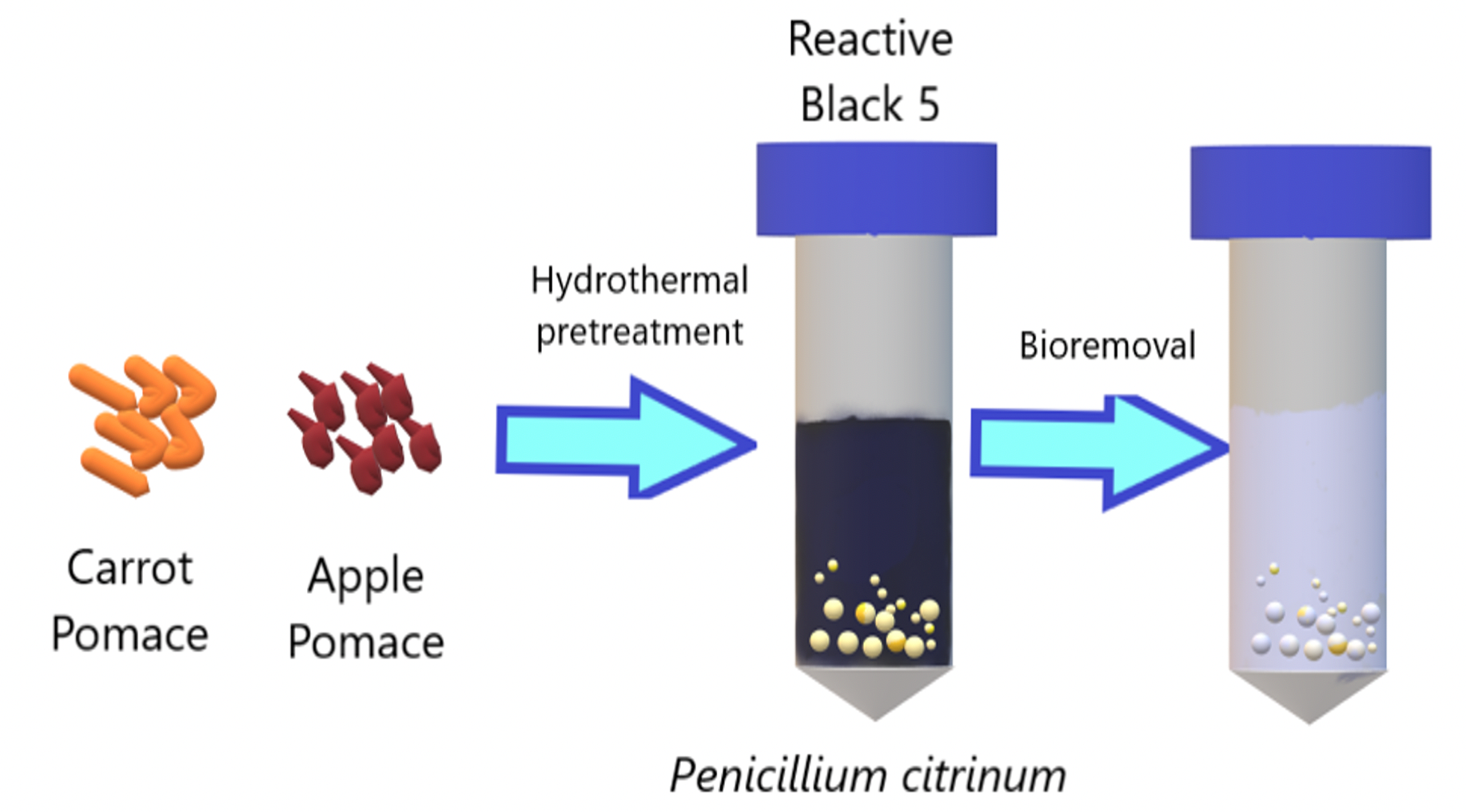
In this study, Reactive Black 5 bioremoval by Penicillium citrinum was investigated. Common agricultural by-products of the food industry, such as apple and carrot pomaces were used as carbon sources. Carrot and apple pomace were pretreated hydrothermally, and the effects of some critical parameters such as pH, initial biomass and dye loadings on Reactive Black 5 bioremoval were investigated. P. citrinum removed 80.87% of the dye in the presence of 16% carrot pomace, pH 5 and 225.9 mg/L initial dye loading, respectively. The maximum specific dye uptake rate was found as 52.92 mg/g under the same conditions. Moreover, notably bioremoval was observed even in high Reactive Black 5 concentrations such as 758.2 mg/L. The results showed that P. citrinum is suitable microorganism for dye removal, and carrot and apple pomaces can be evaluated for microbial growth as a cheap carbon source.
References
- D.A. Yaseen, M. Scholz. Textile dye, Int. J. Environ. Sci. Technol. 16, 1226, (2019).
- K. Murugesan, A. Dhamija, I. H. Nam, Y. M. Kim, Y.S. Chang, Dye Pigment. 75, 184, (2007).
- D. Jager, D. Kupka, M. Vaclavikova, L. Ivanicova, G. Gallios, Chemosphere. 190, 416, (2018).
- L. Ayed, S. Achour, A. Bakhrouf, Water SA. 37, 26, (2011).
- Y. Fu, T. Viraraghavan, Bioresour Technol.79, 262, (2001).
- S. K. Sen, S. Raut, P. Bandyopadhyay P, S. Raut, Fungal Biol Rev. 30, 133, (2016).
- A. Clementz, P. A. Torresi, J. S. Molli, D. Cardell, E. Mammarella, J. C. Yori, LWT. 100, 374, (2019).
- F. Figuerola, M. L. Hurtado, A. M. Estévez, I. Chiffelle, F. Asenjo, Food Chem. 91, 395, (2005).
- V. S. Munagapati, V. Yarramuthi, Y. Kim, K. M. Lee, D. S.Kim, Ecotoxicol Environ Saf. 148, 601, (2018).
- S. E. Karatay, G. Dönmez, Ecol Eng. 73, 224, (2014).
- S. Paul, A. Dutta, Resour Conserv Recycl. 130, 164, (2018).
- E. Palmqvist, B. Hahn-Hägerdal, Bioresour Technol. 74, 25, (2000).
- F. Vendruscolo, P. M. Albuquerque, F. Streit, E. Esposito, J. L. Ninow, Crit Rev Biotechnol. 28, 1, (2008).
- S. Surbhi, V. Rc, R. Deepak, J. Hk, Y. Kk, Int J Chem Stud. 6, 2921, (2018).
- S. Pathania, N. Sharma, S. Handa, Biocatal Biotransformation. 35, 450, (2017).
- R. S. Singh, K. Chauhan, J. Singh, A. Pandey, C. Larroche, Food Technol Biotechnol. 56, 31, (2018).
- S. A. Khan, M. Hamayun, H. Yoon, H. Y. Kim, S. J. Suh, S. K. Hwang, BMC Microbiol. 8, 1, (2008).
- C. Pang, Y. H. Liu, X. H. Cao, M. Li, G. L. Huang, R. Hua, Chem Eng J. 170, 1, (2011).
- Y. Tashiro, H. Ueno, M. Takaba, S. Hayashi, Curr Microbiol. 74, 1114, (2017).
- Y. Gu, P. Ding, Z. Liang, Y. Song, Y. Liu, G. Chen, Fitoterapia. 127, 207, (2018).
- M. R. Deivasigamni, P. Ramalingam, J Adv Chem. 13, 6438, (2017).
- G. L. Miller, Anal Chem. 31, 426, (1959).
- N. R. Aimaretti, C. V. Ybalo, M. L. Rojas, F. J. Plou, J. C. Yori, Bioresour Technol. 123, 727, (2012).
- R. Mutzehilan, S. Yogananth, S. Jayalakshmi, Res J Microbiol. 3, 204 (2008).
- D. Iandolo, A. Amore, L. Birolo, G. Leo, G. Olivieri, V. Faraco, Bioresour Technol. 102, 7603, (2011).
- L.R. Bergsten-Torralba, M. M. Nishikawa, D. F. Baptista, D. P. Magalhães, M. da Silva, Brazilian J Microbiol. 40, 808, (2009).
- B. E: Wang, Y. Y. Hu, L. Xie, K. Peng, Bioresour Technol. 99, 794, (2008).
- M. Gou, Y. Qu, J. Zhou, F. Ma, L. Tan, J Hazard Mater. 170, 314, (2009).
- B. E. Taştan, S. Ertuǧrul, G. Dönmez, Bioresour Technol. 101, 870, (2010).
- C. F. Iscen, I. Kiran, S. Ilhan, J Hazard Mater. 143, 335, (2007).
- H. B. Klinke, L. Olsson, A. B. Thomsen, B. K. Ahring, Biotechnol Bioeng. 81, 737, (2003).
- A. V. Karpe, I. H. Harding, E. A. Palombo, Ind Crops Prod. 59, 228, (2014).
- M. A. Kumar, S. Keerthana, A. Pani, D. Suresh, C. B. Lakshmikantha, M. Seenuvasan, Int J Biomed Eng Technol. 2, 1, (2011).
- Z. Aksu, N. K. Kiliç, S. Ertuǧrul, G. Dönmez, Enzyme Microb Technol. 40, 1167, (2007).


